Calculate the equilibrium constant for the reaction,
H2(g)+CO2(g)⇔H2O(g)+CO(g) at 1395K, if the
equilibrium constants at 1395K for the following are:
2H2O(g)⇔2H2+O2(g) (K1=2.1×10−13)
2CO2(g)⇔2CO(g)+O2(g) (K2=1.4×10−12)
The correct Answer is:2.58
H2(g)+CO2(g)⇔H2O(g)+CO(g).
2H2O(g)⇔2H2(g)+O2(g)K1=2.1×10−13 ....(1)
2CO2(g)⇔2CO(g)+O2(g)K2=1.4×10−12 ....(2)
12eq.(2)−12eq.(1)
CO2(g)−H2O(g)⇔CO(g)+12O2(g).
CO2(g)+H2(g)⇔H2O(g)+CO(g).
12[eq.(2)−eq.(1)]⇒K=(K2K1)1/2=(1.4×10−122.1×10−13)1/2=(142.1)1/2=2.58.
Topper's Solved these Questions
- CHEMICAL EQUILIBRIUMBook:RESONANCEChapter:CHEMICAL EQUILIBRIUMExercise:Exercise-1 (Part-2)
Explore 88 Videos
- CHEMICAL EQUILIBRIUMBook:RESONANCEChapter:CHEMICAL EQUILIBRIUMExercise:Exercise-2 (Part-1)
Explore 28 Videos
- CHEMICAL EQUILIBRIUMBook:RESONANCEChapter:CHEMICAL EQUILIBRIUMExercise:Board Level Exercise
Explore 33 Videos
- CHEMICAL BONDINGBook:RESONANCEChapter:CHEMICAL BONDINGExercise:Inorganic chemistry (Chemistry Bonding)
Explore 49 Videos
- D & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS Book:RESONANCEChapter:D & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS Exercise:Match the column
Explore 1 Video
Similar Questions
A reversible reaction is one which
View SolutionThe equilibrium constant (Kp) for the reaction, PCl5(g)⇔PCl3(g)+Cl2(g) is 16. If the volume of the container is reduced to half of its original volume, the value of Kp for the reaction at the same temperature will be:
View SolutionThe equilibrium constant expression for a gas reaction is :
Kc=[NH3]4[O2]5[NO]4[H2O]6
Write the balanced chemical equation corresponding to this expression.
View SolutionWhich is correct order ofr net dipole moment :
View SolutionMatch the reactions in Column I with the types of products/the use of products in Column II.
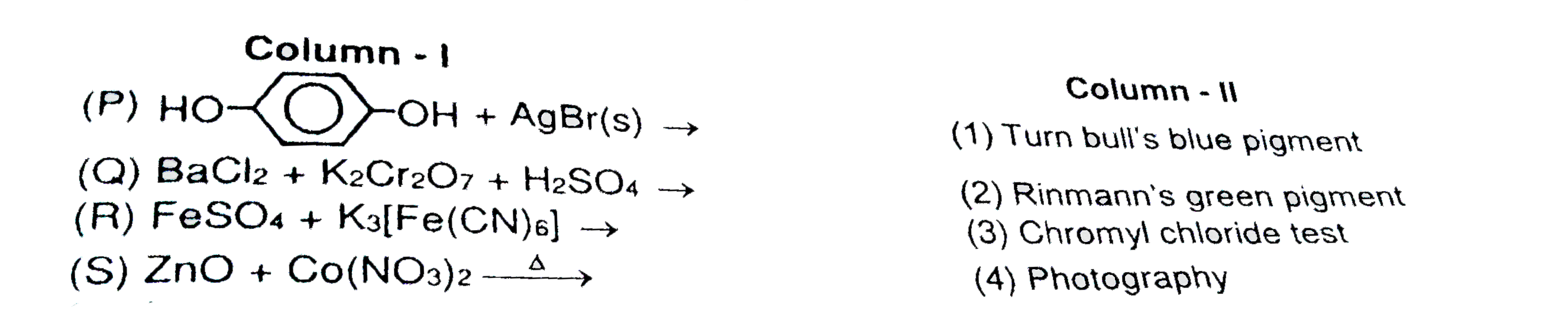
View SolutionThe relation between Kp and Kc is Kp=Kc(RT)Δn unit of Kp=(atm)Δn, unit of Kc=(molL−1)Δn
The equilibrium constant of the following reactions at 400K are given:
2H2O(g)⇔2H2(g)+O2(g),K1=3.0×10−13
2CO2(g)⇔2CO(g)+O2(g),K2=2×10−12
Then, the equilibrium constant K for the reaction
H2(g)+CO2(g)⇔CO(g)+H2O(g)
is
View SolutionCalculate the equilibrium constant for the reaction, H_(2(g))+CO_(2(g))hArrH_(2)O_((g))+CO_((g)) at 1395 K , if the equilibrium constants at 1395 K for the following are: 2H_(2)O_((g))hArr2H_(2)+O_(2(g)) ( K_(1)=2.1xx10^(-13) ) 2CO_(2(g))hArr2CO_((g))+O_(2(g)) ( K_(2)=1.4xx10^(-12) )
View SolutionIf 2H_(2)O_((g))hArr2H_(2(g))+O_(2(g)), K_(1)=2.0xx10^(-13) 2CO_(2(g))hArr2CO_((g))+O_(2(g)), K_(2)=7.2xx10^(-12) Find the equilibrium constant for the reaction CO_(2(g))+H_(2(g))hArrCO_((g))+H_(2)O_((g))
View SolutionFor the following three reaction 1, 2 and 3, equilibrium constants are given: (1) CO_((g)) + H_(2)O_((g))hArrCO_(2(g)) + H_(2(g)), K_(1) (2) CH_(4(g)) + H_(2)O_((g))hArrCO_((g)) + 3H_(2(g)), K_(2) (3) CH_(4(g)) + 2H_(2)O_((g))hArrCO_(2(g)) + 4H_(2(g)), K_(3) Which of the following relations is correct ?
View SolutionFor the reaction N2(g)+O2(g)⇔2NO(g), the equilibrium constant is K1. The equilibrium constant is K2 for the reaction
2NO(g)+O2⇔2NO2(g)
What is K for the reaction
NO2(g)⇔12N2(g)+O2(g)?
View SolutionThe equilibrium constant for the following reactions at 1400 K are given. 2H_(2)O(g)hArr2H_(2)(g) + O_(2)(g) , K_(1)=2.1×x10^(-13) 2CO_(2)(g) hArr2CO(g)+O_(2)(g),K_(2) = 1.4 x× 10^(-12) Then, the equilibrium constant K for the reaction H_(2)(g) + CO_(2)(g)hArrCO(g) + H_(2)O(g) is
View SolutionThe equilibrium constant for the reaction H_(2)O(g)+CO(g)hArrH_(2)(g)+CO_(2)(g) is 0.44 at 1660 K. The equilibrium constant for the reaction 2H_(2)(g)+2CO_(2)(g)hArr 2CO(g)+2H_(2)O(g) at 1660 K is equal to
View SolutionWrite the equilibrium constant for the following
(i) H2O2(g)⇔H2O(g)+1/2O2(g)
(ii) CO(g)+H2O(g)⇔CO2(g)+H2(g)
(iii) N2O4(g)⇔2NO2(g)
View SolutionWrite the equilibrium constant for the following (i) H_(2)O_(2(g))hArrH_(2)O_((g))+1//2O_(2(g)) (ii) CO_((g))+H_(2)O_((g))hArrCO_(2(g))+H_(2(g)) (iii) N_(2)O_(4(g))hArr2NO_(2(g))
View SolutionEquilibrium constant for the following reactions at 1200 K are given : 2H_(2)O_((g))iff2H_(2(g))+O_(2(g)),K_(1)=6.4xx10^(-8) 2CO_(2(g))iff2CO_((g))+O_(2(g)),K_(2)=1.6xx10^(-6) The equilibrium constant for the reaction H_(2(g))+CO_(2(g))iffCO_((g))+H_(2)O_((g)) at 1200 K will be
View SolutionWrite the equilibrium constant for the following
(i) H2O2(g)⇔H2O(g)+1/2O2(g)
(ii) CO(g)+H2O(g)⇔CO2(g)+H2(g)
(iii) N2O4(g)⇔2NO2(g)
View Solution
