In the Haber process for the industrial manufacturing of ammonia involving the reaction, N2(g)+3H2(g)⇔2NH3(g) at 200 atm pressure in the presence of a catalyst, a temperature of about 500∘C is used. This considered as optimum temperature for the process because :
yield is maximum at this temperature
catalyst is active only at this temperature
energy needed for the reaction is easily obtained at this temperature
rate of the catalytic reaction is fast enough while the yield is also appreciable for this exothermic reaction at this temperature.
The correct Answer is:D
Step by step video, text & image solution for In the Haber process for the industrial manufacturing of ammonia involving the reaction, N_2(g)+3H_2(g)hArr 2NH_3(g) at 200 atm pressure in the presence of a catalyst, a temperature of about 500^@C is used. This considered as optimum temperature for the process because : by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
- CHEMICAL EQUILIBRIUMBook:RESONANCEChapter:CHEMICAL EQUILIBRIUMExercise:Exercise-2 (Part-2)
Explore 23 Videos
- CHEMICAL EQUILIBRIUMBook:RESONANCEChapter:CHEMICAL EQUILIBRIUMExercise:Exercise-2 (Part-3)
Explore 34 Videos
- CHEMICAL EQUILIBRIUMBook:RESONANCEChapter:CHEMICAL EQUILIBRIUMExercise:Exercise-1 (Part-2)
Explore 88 Videos
- CHEMICAL BONDINGBook:RESONANCEChapter:CHEMICAL BONDINGExercise:Inorganic chemistry (Chemistry Bonding)
Explore 49 Videos
- D & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS Book:RESONANCEChapter:D & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS Exercise:Match the column
Explore 1 Video
Similar Questions
How many of the following reactions are homogenous reversible reactions?
(1) CH3COOH(l)+C2H5OH(l)⇔CH3COOC2H5(l)+H2O(l)
(2) C(s)+CO2(g)⇔CO(g)+H2O(g)
(3) H2(g)+CO2(g)⇔CO(g)+H2O(g)
(4) CO(g)+CI2(g)⇔COCI2(g)
(5) NH4HSs⇔NH3(g)+H2S(g)
(6) CaCO3(s)⇔CaO(s)+CO2(s)
(7) N2(g)O2(g)⇔2NO(g)
(8) CO2(g)+C(s)⇔2CO(g)
(9) SO2+NO2(g)⇔SO3(g)+NO(g)
(10) NO(g)+12Br2(l)⇔2NOBr(g)
View SolutionWhich of the following is correct about the chemical equilibrium ?
View SolutionA reversible reaction is one which
View SolutionWhich is correct order ofr net dipole moment :
View SolutionMatch the reactions in Column I with the types of products/the use of products in Column II.
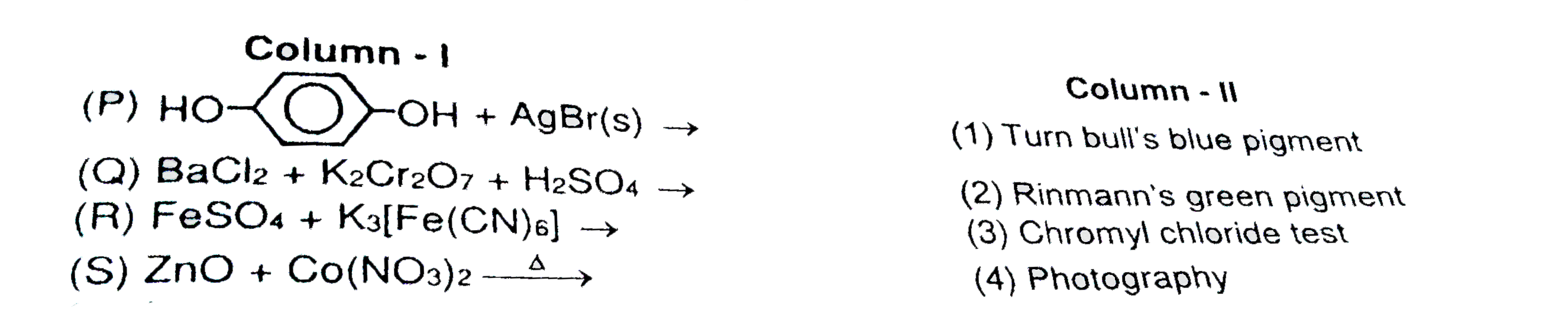
View SolutionFor the synthesis of ammonia, K_c is 1.2 at 375^@C,N_2(g)+3H_2(g)hArr 2NH_3(g) What is K_p at this temperature ?
View SolutionIn the Haber process for the industrial manufacturing of ammonia involving the reaction, N_2(g)+3H_2(g)hArr 2NH_3(g) at 200 atm pressure in the presence of a catalyst, a temperature of about 500^@C is used. This considered as optimum temperature for the process because :
View SolutionFor a gas reaction , 3H_2(g)+N_2(g)hArr 2NH_3(g) , the partial pressures of H_2 and N_2 are 0.4 and 0.8 atmosphere , respectively at equilibrium . The total pressure of the entire system is 2.8 atmosphere. What will be the value of k_p if all the concentrations are given in atmospheres ? N_2(g) +3H_2(g)hArr 2NH_3(g)
View Solutionहैबर प्रक्रम (Haber's Process) के द्वारा अमोनिया के निर्माण में N2(g)+3H2(g)⇔2NH3(g)+92.3 किलोजूल निम्न में से कौन-सी शर्त प्रतिकूल है?
View SolutionFor the reaction at 127^@ C N_2(g) + 3H_2(g) hArr 2NH_3(g) the partial pressures of N_2 and H_2 are 0.80 and 0.40 atm respectively at equilibrium. The total pressure of the system is 2.80 atm. Calculate K_p and K_c for the reaction.
View SolutionAt 207^@C , for the reaction : N_2(g) + 3H_2(g) hArr 2NH_3(g) it is found that at equilibrium, the mixture contains 52% of ammonia. If the pressure of the mixture is 10 atm, calculate K_p and K_c for the reaction.
View SolutionThe formation of ammonia from N_2(g) and H_2(g) is a reversible reaction N_2(g) + 3H_2(g) hArr 2NH_3(g) + "Heat" What is the effect of increase of temperature on this equilibrium reaction
View SolutionK_C/K_P for the reaction, N_2(g) + 3H_2(g) hArr 2NH_3(g) is ……………………. .
View Solutionअभिक्रिया N2(g)+3H2(g)⇔2NH3(g),ΔH=−93.6 kJ में अमोनिया का उत्पादन नहीं बढ़ेगा, जब-
View SolutionApply law of mass action to the process, N_2(g) + 3H_2 (g) hArr 2NH_3 (g).
View SolutionFor the reaction, N_2[g] + 3H_2[g] hArr 2NH_3[g] , Delta H = …
View Solution
