Hofmann rearrangement
In the Hofmann rearrangement an unsubstitued amide is treated with sodium hydroxide and bromine to give a primary amine that has one carbon lesser than starting amide.
Gneral reaction: 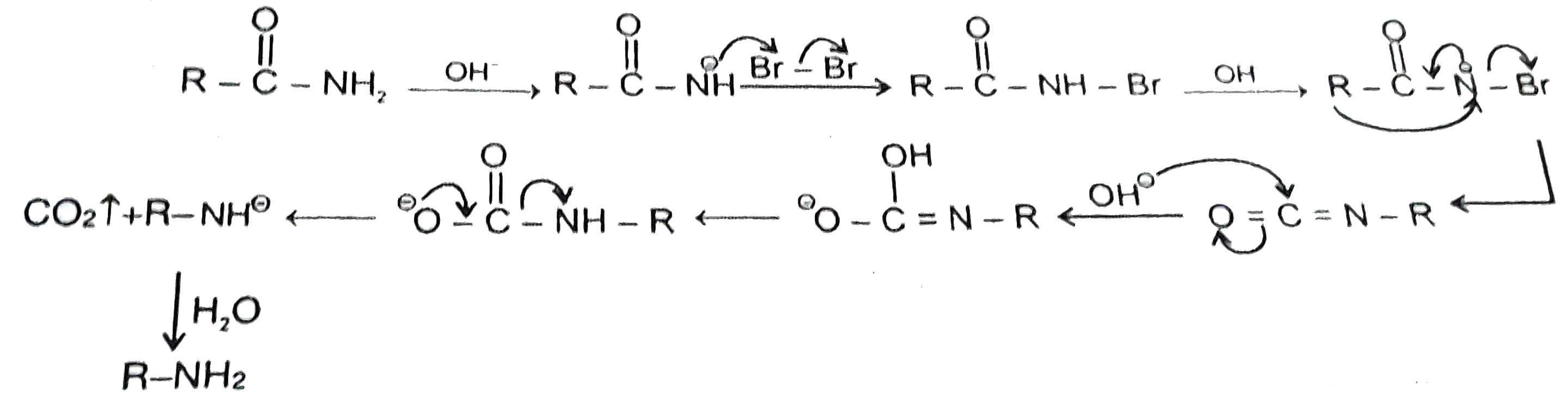
If the migrating group is chiral then its configuration is retained. Electron releasing effects in the migrating group increase reactivity of Hofmann rearangement.
Which of the following compound(s) cannot give Hoffman rearrangement:
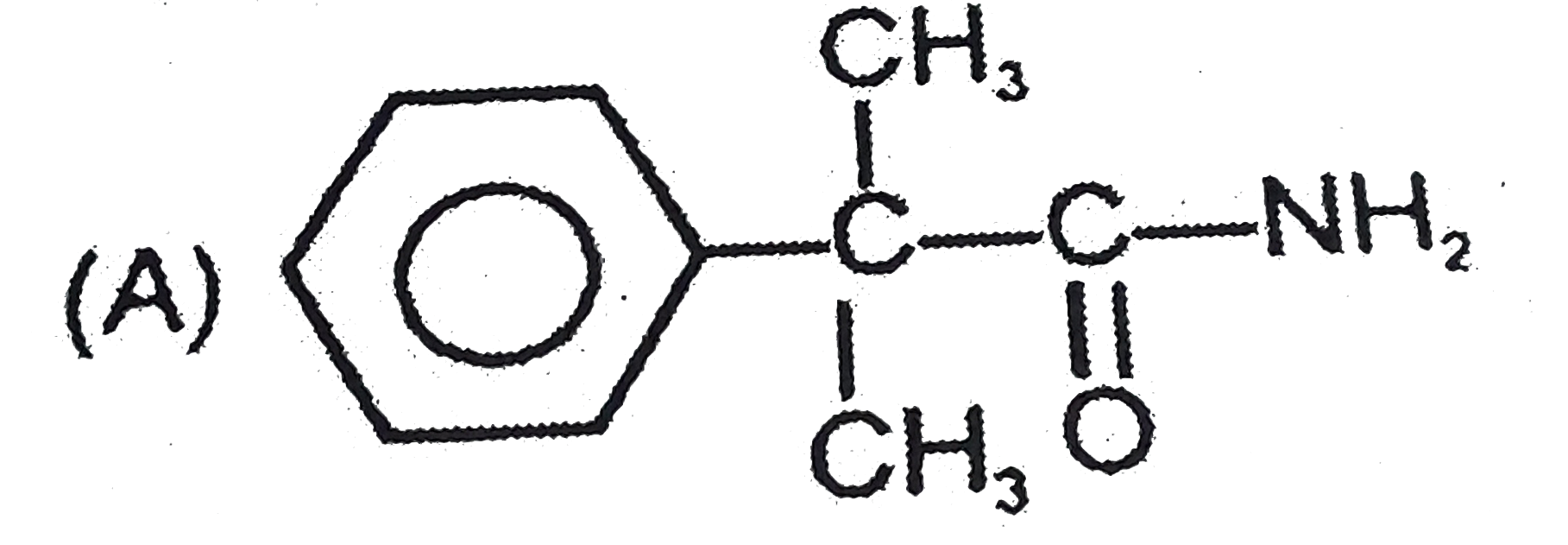
CH3−CH2−O∣∣C−NH−Ph
Ph−O∣∣C−NH2
The correct Answer is:B
Similar Questions
Hofmann rearrangement
In the Hofmann rearrangement an unsubstitued amide is treated with sodium hydroxide and bromine to give a primary amine that has one carbon lesser than starting amide.
Gneral reaction: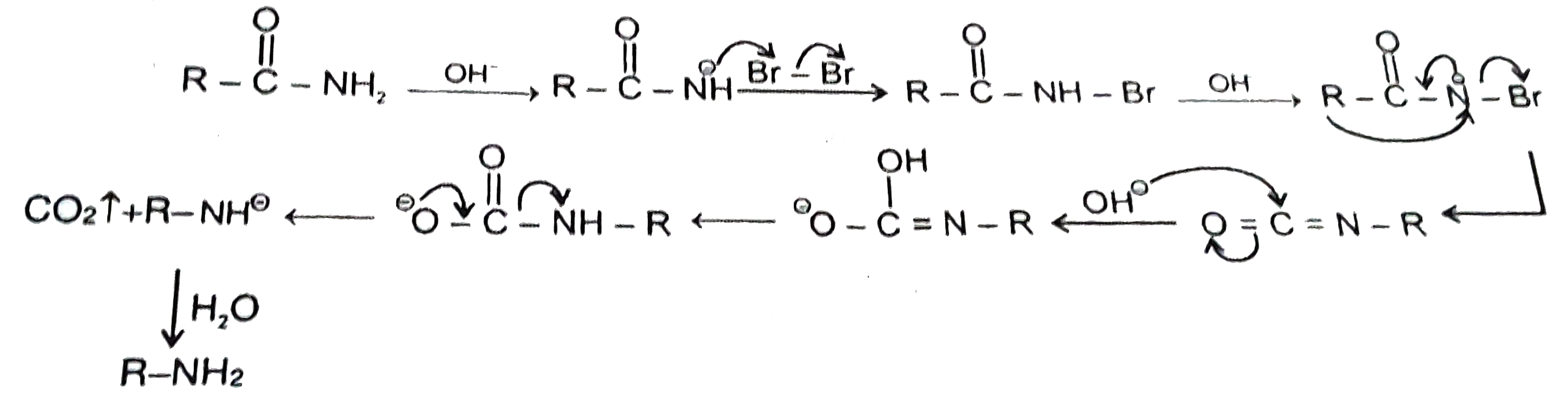
If the migrating group is chiral then its configuration is retained. Electron releasing effects in the migrating group increase reactivity of Hofmann rearangement.
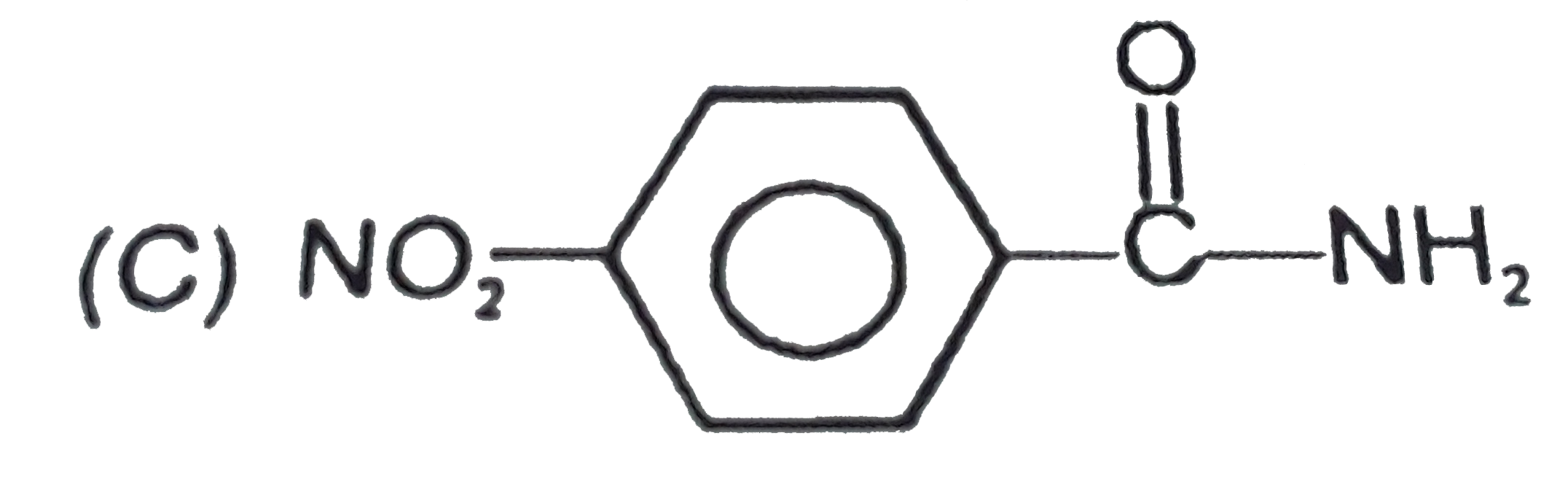
Arrange the following amides according to their relative reactivity when reacted with Br2 in excess of strong base:

View SolutionStatement-1 : Electrons releasing group at para position of a migrating aryl group enhances. The rate of reaction in Hofmann's preparation of primary amine from amides.
Statement-2:
View Solution
Knowledge Check
Hofmann rearrangement
In the Hofmann rearrangement an unsubstituted amide is treated with sodium hydroxide and bromide to give a primary amine that has one carbon lesser than starting amide.
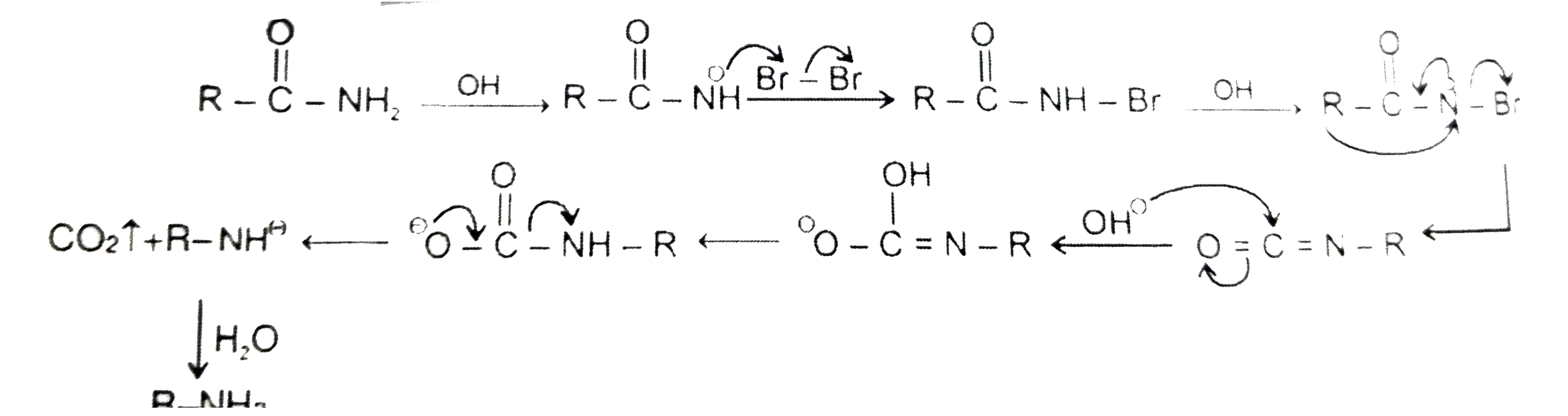
In the migrating group is chiral then is configuration is retained. Electron releasing effects in the migrating group increases reactivity of Hofmann rearrangement.
Which of the following compounds (s) cannot give Hofmann rearrangement :A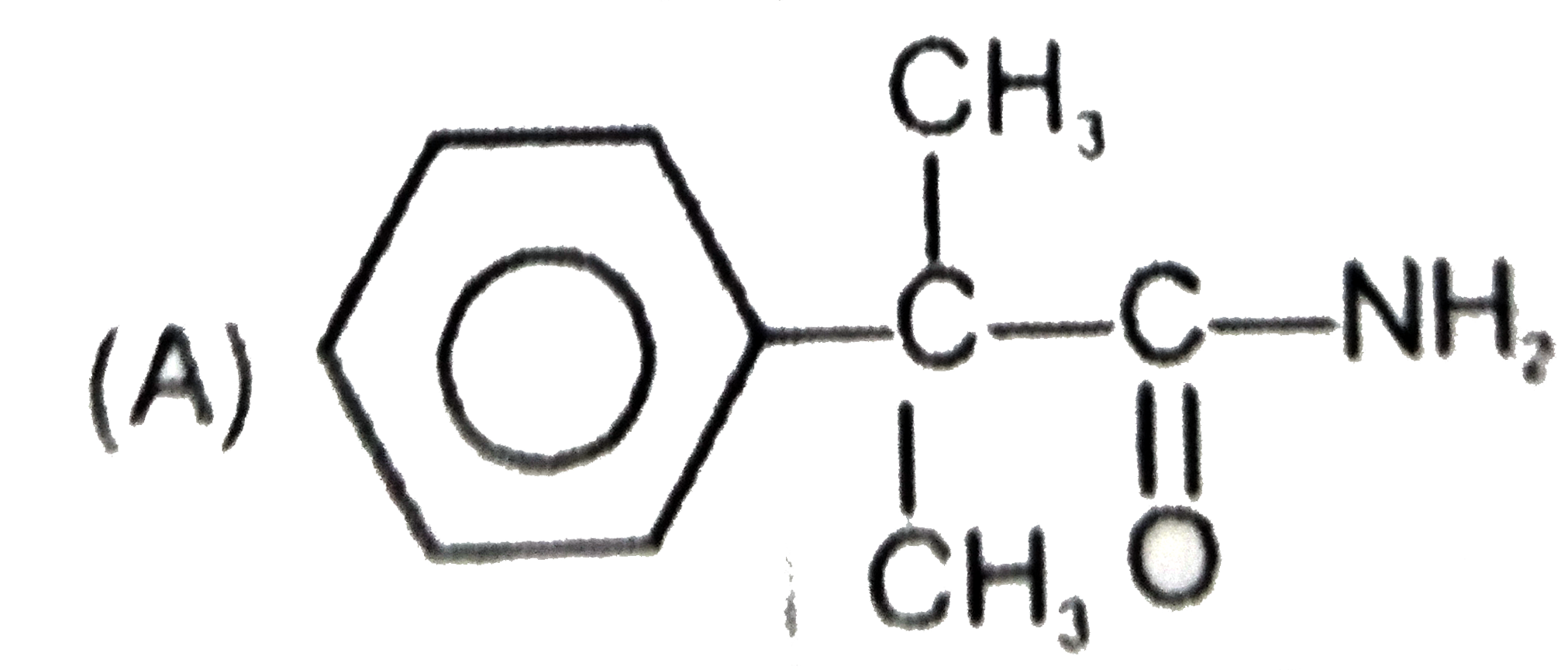 BCH3−CH2−O∣∣C−NH−PhC
BCH3−CH2−O∣∣C−NH−PhC DPh−O∣∣C−NH2
DPh−O∣∣C−NH2Hofmann rearrangement
In the Hofmann rearrangement an unsubstitued amide is treated with sodium hydroxide and bromine to give a primary amine that has one carbon lesser than starting amide.
Gneral reaction:
If the migrating group is chiral then its configuration is retained. Electron releasing effects in the migrating group increase reactivity of Hofmann rearangement.
Arrange the following amides according to their relative reactivity when reacted with Br2 in excess of strong base:
 AIV>I>II>IIIBII>I>III>IVCII>IV>III>IDII>I>IV>III
AIV>I>II>IIIBII>I>III>IVCII>IV>III>IDII>I>IV>IIIHofmann rearrangement
In the Hofmann rearrangement an unsubstituted amide is treated with sodium hydroxide and bromide to give a primary amine that has one carbon lesser than starting amide.
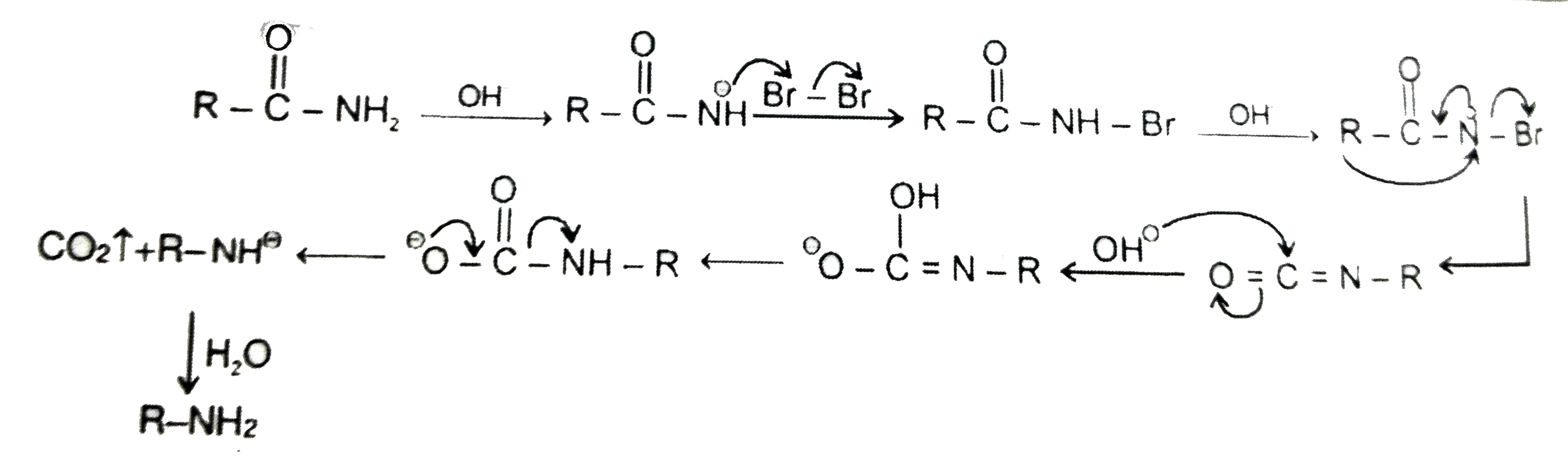
In the migrating group is chiral then is configuration is retained. Electron releasing effects in the migrating group increases reactivity of Hofmann rearrangement.
Arrange the following amides according to their relative when reacted with Br2 in excess of strong base :
 AIV>I>II>IIIBII>I>III>IVCII>IV>III>IDII>I>IV>III
AIV>I>II>IIIBII>I>III>IVCII>IV>III>IDII>I>IV>III
Hofmann rearrangement
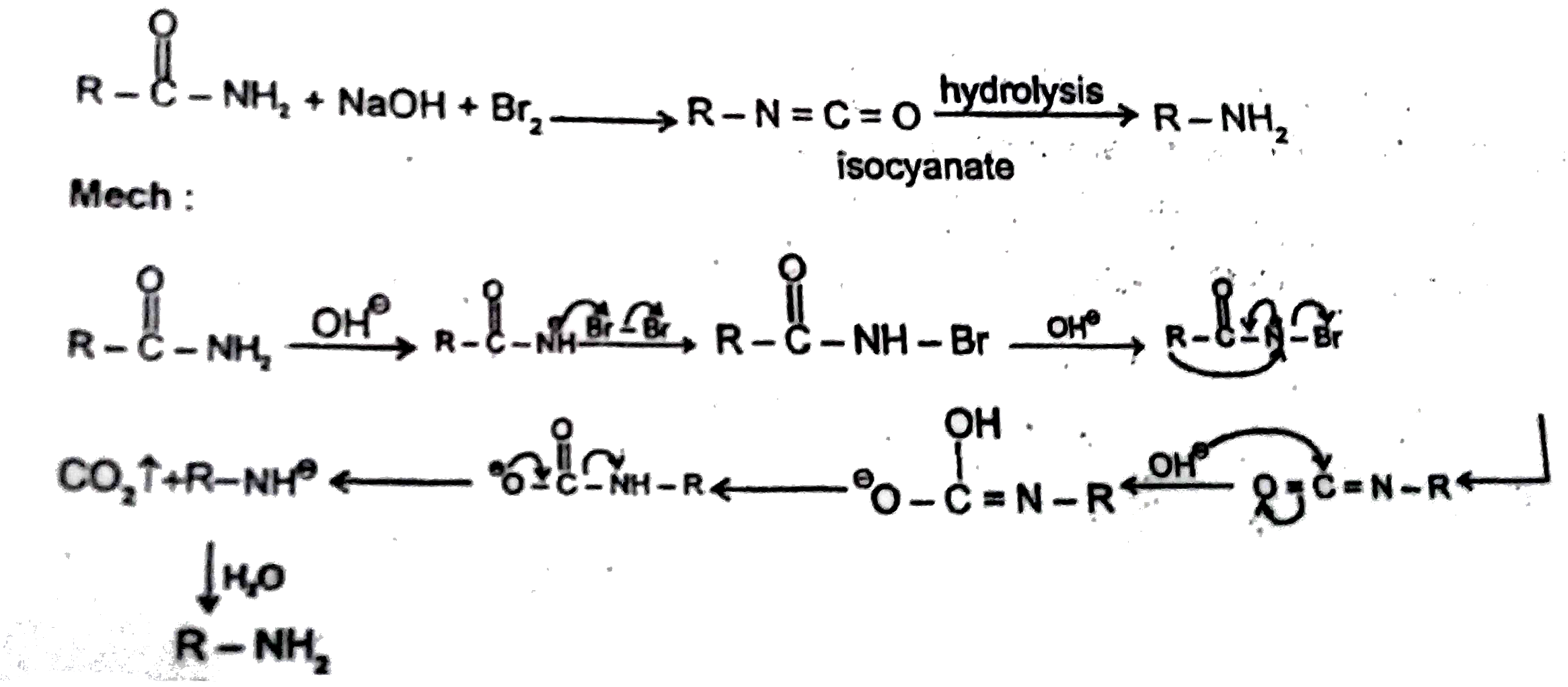
In the Hoffmann rearrangement an unsubstituted amide is treated with sodium hydroxide and bromine to give a primary amine that has one carbon lesser than starting amide.
R−O∣∣C−NH2+NaOH+Br2→R−N=C=Oisocyanatehydrolysis−−−−−→R−NH2
Mech :
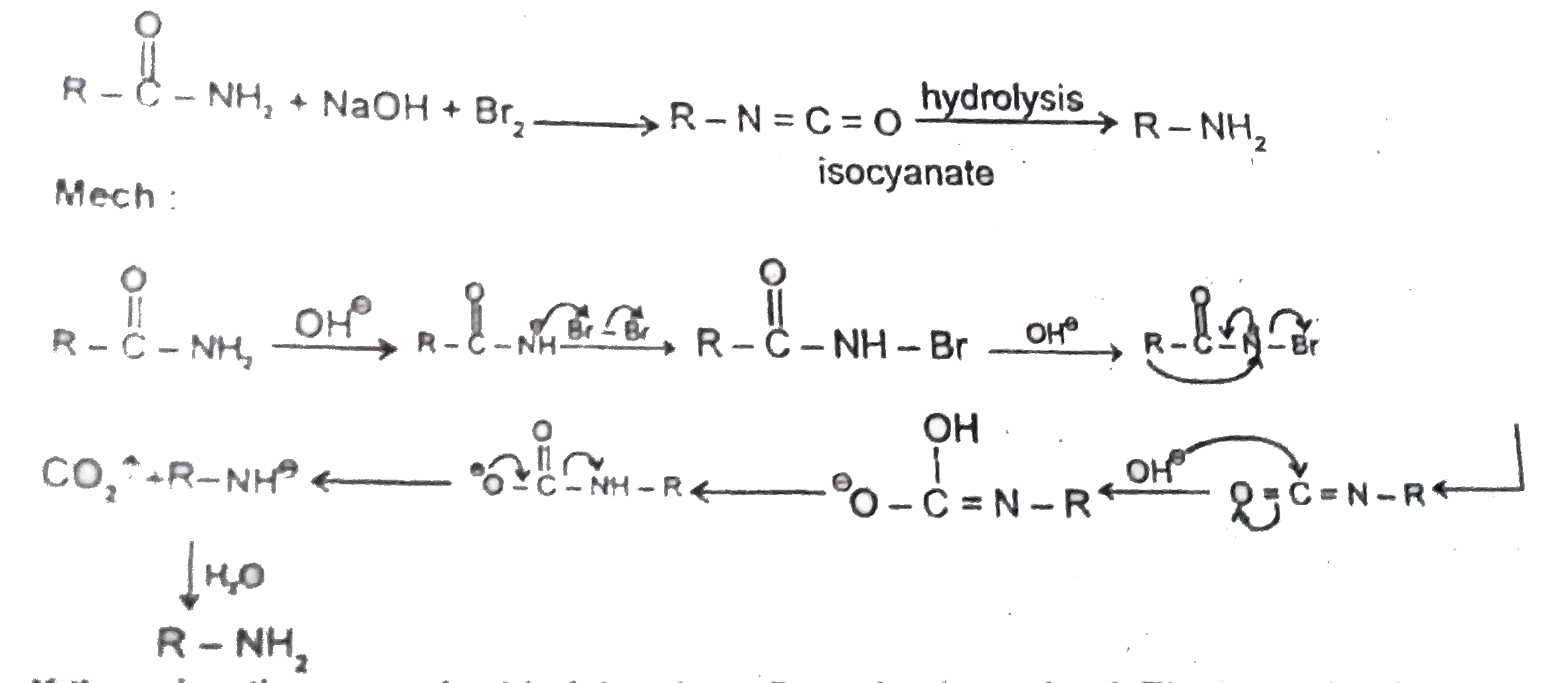
If the migrating group is chiral then its configuration is retained . Electron releasing effects in the migrating group increases reactivity of Hofmann rearrangement.
Which of the following compound cannot give Hofmann rearrangement :
View SolutionHofmann rearrangement
In the Hoffmann rearrangement an unsubstituted amide is treated with sodium hydroxide and bromine to give a primary amine that has one carbon lesser than starting amide.
R−O∣∣C−NH2+NaOH+Br2→R−N=C=Oisocyanatehydrolysis−−−−−→R−NH2
Mech :
If the migrating group is chiral then its configuration is retained . Electron releasing effects in the migrating group increases reactivity of Hofmann rearrangement.
Arrange the following amides according to their relative reactivity when react with Br2 in excess of strong base
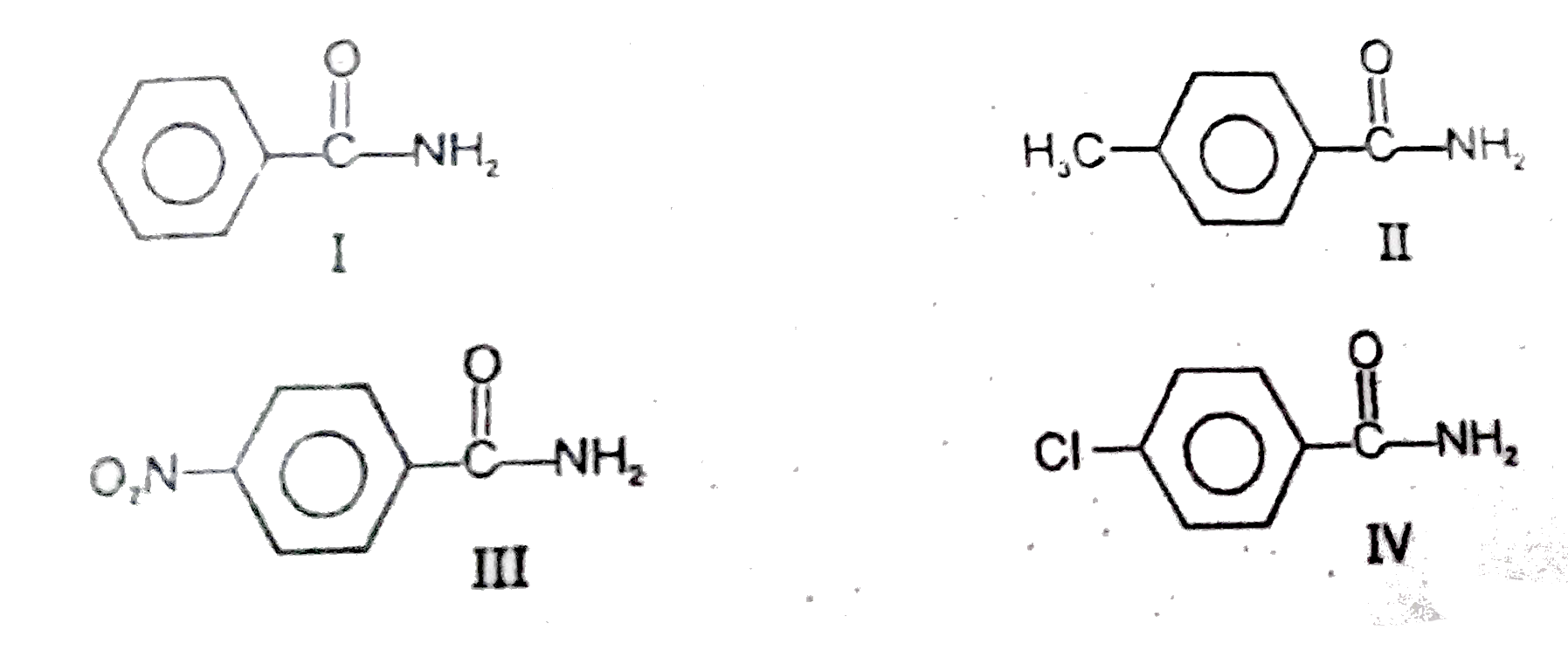
View SolutionHofmann rearrangement
In the Hoffmann rearrangement an unsubstituted amide is treated with sodium hydroxide and bromine to give a primary amine that has one carbon lesser than starting amide.
R−O∣∣C−NH2+NaOH+Br2→R−N=C=Oisocyanatehydrolysis−−−−−→R−NH2
Mech :
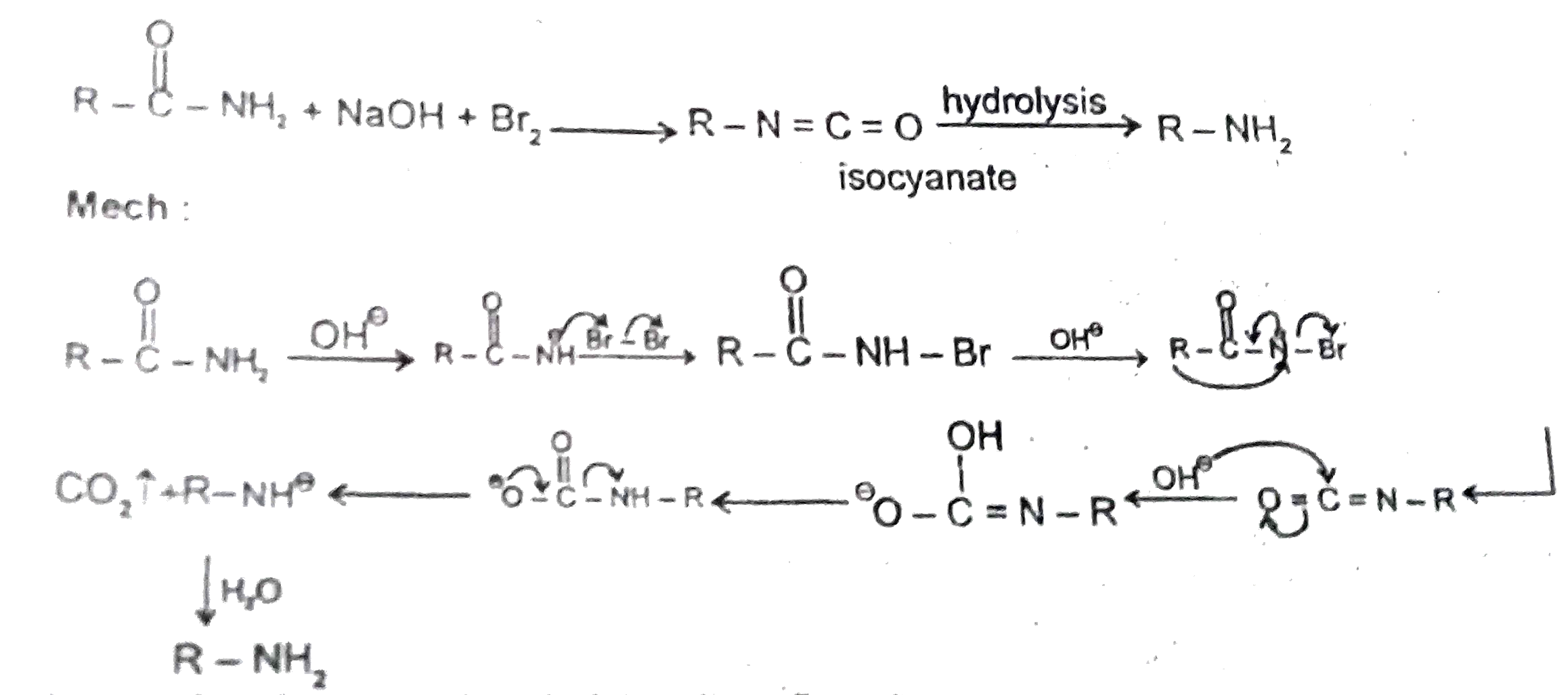
If the migrating group is chiral then its configuration is retained . Electron releasing effects in the migrating group increases reactivity of Hofmann rearrangement.
H3C−H2C−∗C∣CH3(R)H−O∣∣C−NH2+CH3−CH2−D∣C∣CH3(S)−O∣∣C−15NH2Br2/KOH−−−−−−→Products
View SolutionStatement-1 : Electrons releasing group at para position of a migrating aryl group enhances. The rate of reaction in Hofmann's preparation of primary amine from amides.
Statement-2:
View SolutionThe conversion of an amide into an amine with one carbon atom less by the action of alkaline hydrohalite is known as Hofmann bromamide rearrangement:
RCONH2Br2/KOH−−−−−−→R−NH2
The most important feature of the reaction is the arrangement of N–bromamide anion to isocyanate :
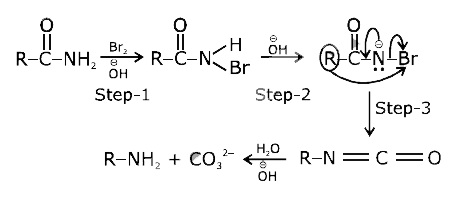
Which of the following can undergo Hofmann bromamide reaction most easily ?
View Solution

