Hofmann rearrangement
In the Hofmann rearrangement an unsubstitued amide is treated with sodium hydroxide and bromine to give a primary amine that has one carbon lesser than starting amide.
Gneral reaction: 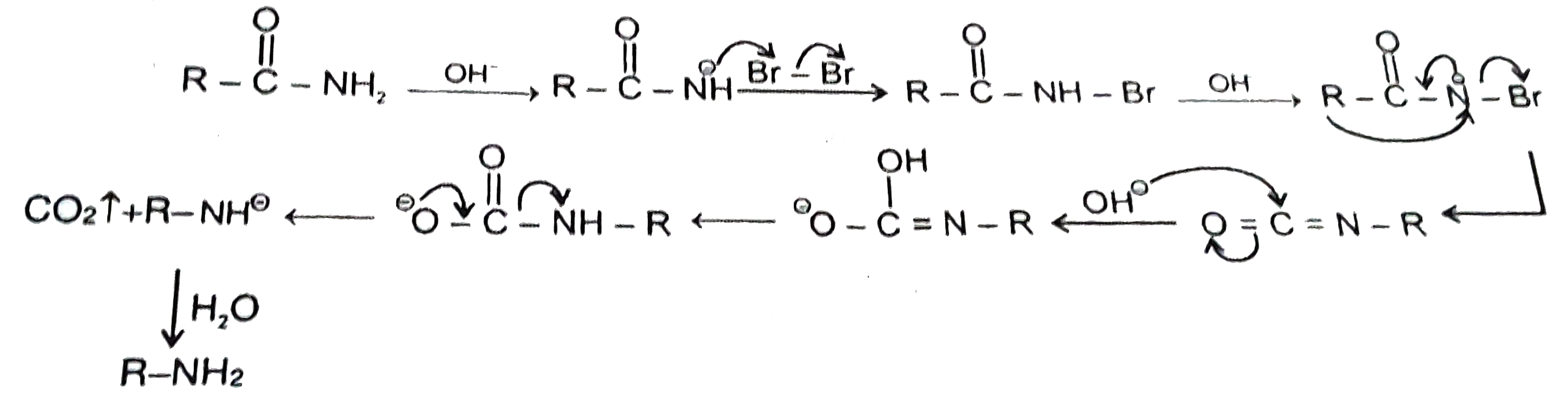
If the migrating group is chiral then its configuration is retained. Electron releasing effects in the migrating group increase reactivity of Hofmann rearangement.
Arrange the following amides according to their relative reactivity when reacted with Br2 in excess of strong base:

IV>I>II>III
II>I>III>IV
II>IV>III>I
II>I>IV>III
The correct Answer is:D
Step by step video solution for Hofmann rearrangement In the Hofmann rearrangement an unsubstitued amide is treated with sodium hydroxide and bromine to give a primary amine that has one carbon lesser than starting amide. Gneral reaction: If the migrating group is chiral then its configuration is retained. Electron releasing effects in the migrating group increase reactivity of Hofmann rearangement. Arrange the following amides according to their relative reactivity when reacted with Br_(2) in excess of strong base: by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023টপার এই প্রশ্নগুলো সমাধান করেছে
- AROMATIC COMPOUNDS BOOK - RESONANCE ENGLISHCHAPTER - AROMATIC COMPOUNDS EXERCISE - Exercise -3 Part-I
36 Videos
- AROMATIC COMPOUNDS BOOK - RESONANCE ENGLISHCHAPTER - AROMATIC COMPOUNDS EXERCISE - PART-II JEE MAIN
19 Videos
- AROMATIC COMPOUNDS BOOK - RESONANCE ENGLISHCHAPTER - AROMATIC COMPOUNDS EXERCISE - PART-III ONE OR MORE THAN ONE OPTION CORRECT TYPE
10 Videos
- ATOMIC STRUCTUREBOOK - RESONANCE ENGLISHCHAPTER - ATOMIC STRUCTUREEXERCISE - ORGANIC CHEMISTRY(Fundamental Concept )
16 Videos
সম্পর্কিত প্রশ্ন
Assertion : In strongly acidic solution, aniline becomes less reactive towards electrophilic reagents .
Reason : Due to protonation of amino group the lone pair of electrons on nitrogen is not available for donation to benzene ring by resonance .
View SolutionPicric acid is:
View SolutionWhich of the following can decolourise bromine water solution?
View SolutionThe magnetic moment of .25Mn in ionic state is √15B.M, then Mn is in:
View SolutionHofmann rearrangement In the Hoffmann rearrangement an unsubstituted amide is treated with sodium hydroxide and bromine to give a primary amine that has one carbon lesser than starting amide. R-oversetoverset(O)(||)C-NH_2+NaOH+Br_2toR-N=underset("isocyanate")(C=O)overset("hydrolysis")toR-NH_2 Mech : If the migrating group is chiral then its configuration is retained . Electron releasing effects in the migrating group increases reactivity of Hofmann rearrangement. Which of the following compound cannot give Hofmann rearrangement :
View SolutionHofmann rearrangement In the Hoffmann rearrangement an unsubstituted amide is treated with sodium hydroxide and bromine to give a primary amine that has one carbon lesser than starting amide. R-oversetoverset(O)(||)C-NH_2+NaOH+Br_2toR-N=underset("isocyanate")(C=O)overset("hydrolysis")toR-NH_2 Mech : If the migrating group is chiral then its configuration is retained . Electron releasing effects in the migrating group increases reactivity of Hofmann rearrangement. Arrange the following amides according to their relative reactivity when react with Br_2 in excess of strong base
View SolutionHofmann rearrangement
In the Hoffmann rearrangement an unsubstituted amide is treated with sodium hydroxide and bromine to give a primary amine that has one carbon lesser than starting amide.
R−O∣∣C−NH2+NaOH+Br2→R−N=C=Oisocyanatehydrolysis−−−−−→R−NH2
Mech :
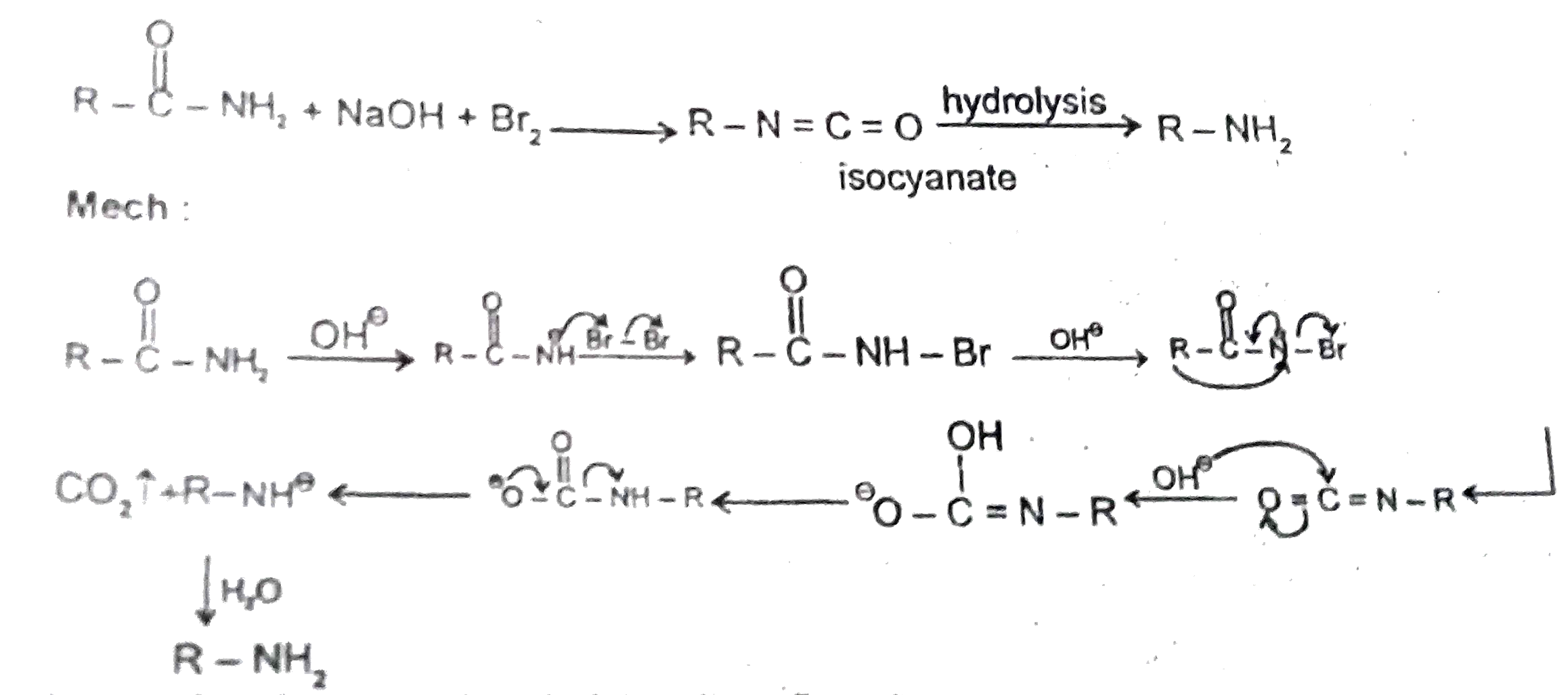
If the migrating group is chiral then its configuration is retained . Electron releasing effects in the migrating group increases reactivity of Hofmann rearrangement.
H3C−H2C−∗C∣CH3(R)H−O∣∣C−NH2+CH3−CH2−D∣C∣CH3(S)−O∣∣C−15NH2Br2/KOH−−−−−−→Products
View SolutionWhich of the following amides will not undergo Hofmann bromamide reaction?
View SolutionWhich amide will be most reactive for Hofmann- bromamide rearrangement?
View SolutionHofmann rearrangement
In the Hofmann rearrangement an unsubstitued amide is treated with sodium hydroxide and bromine to give a primary amine that has one carbon lesser than starting amide.
Gneral reaction: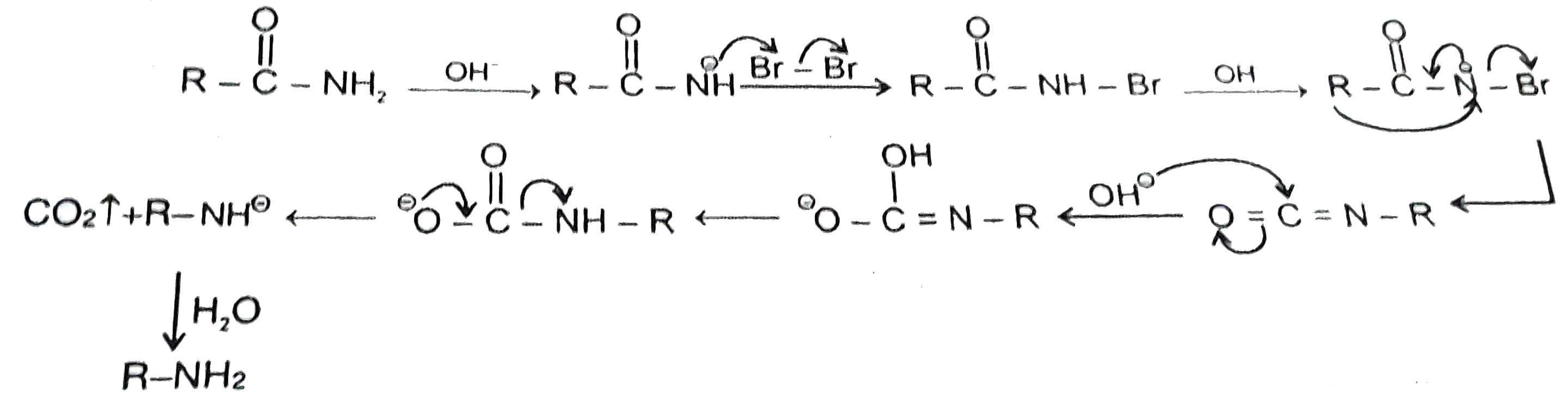
If the migrating group is chiral then its configuration is retained. Electron releasing effects in the migrating group increase reactivity of Hofmann rearangement.
Which of the following compound(s) cannot give Hoffman rearrangement:
View SolutionHofmann rearrangement
In the Hofmann rearrangement an unsubstitued amide is treated with sodium hydroxide and bromine to give a primary amine that has one carbon lesser than starting amide.
Gneral reaction:
If the migrating group is chiral then its configuration is retained. Electron releasing effects in the migrating group increase reactivity of Hofmann rearangement.
Arrange the following amides according to their relative reactivity when reacted with Br2 in excess of strong base:

View SolutionHofmann rearrangement
In the Hofmann rearrangement an unsubstituted amide is treated with sodium hydroxide and bromide to give a primary amine that has one carbon lesser than starting amide.
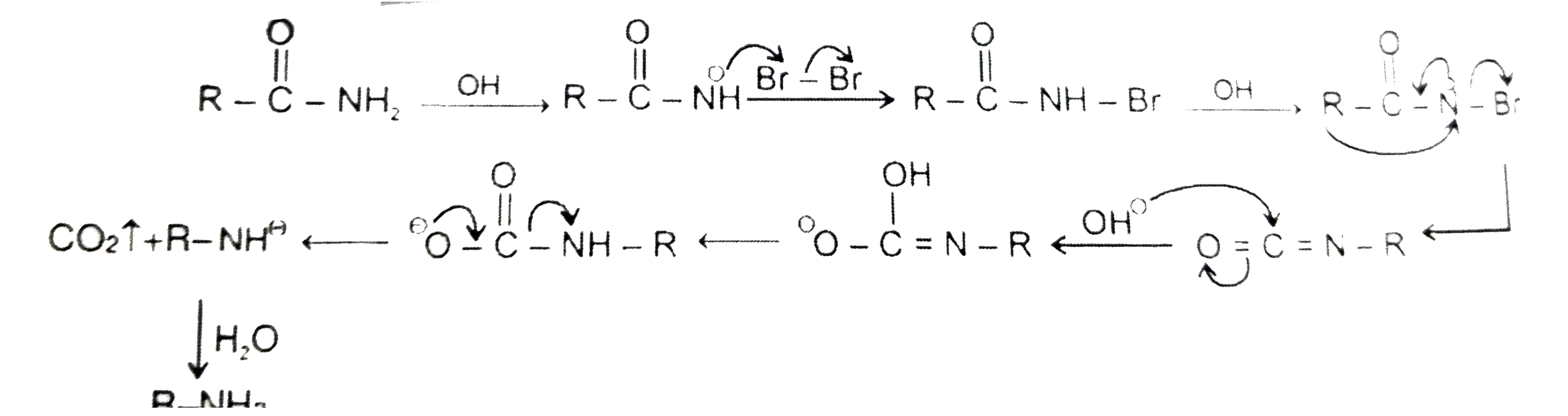
In the migrating group is chiral then is configuration is retained. Electron releasing effects in the migrating group increases reactivity of Hofmann rearrangement.
Which of the following compounds (s) cannot give Hofmann rearrangement :
View SolutionHofmann rearrangement In the Hofmann rearrangement an unsubstituted amide is treated with sodium hydroxide and bromide to give a primary amine that has one carbon lesser than starting amide. In the migrating group is chiral then is configuration is retained. Electron releasing effects in the migrating group increases reactivity of Hofmann rearrangement. Arrange the following amides according to their relative when reacted with Br_(2) in excess of strong base :
View SolutionWhich of the following amides will not undergo Hofmann bromamide reaction ?
View SolutionOne of the following amides will not undergo Hofmann bromamide reaction:
View Solution

