Ionization enthalpy of an atom is equal to
electron gain enthalpy of the cation
electronegativity of the ion
ionization enthalpy of the cation
none of these
The correct Answer is:A
Step by step video, text & image solution for Ionization enthalpy of an atom is equal to by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- PERIODIC PROPERTIESBOOK - VK JAISWALCHAPTER - PERIODIC PROPERTIESEXERCISE - Level 2
102 Videos
- PERIODIC PROPERTIESBOOK - VK JAISWALCHAPTER - PERIODIC PROPERTIESEXERCISE - MATCH THE COLUMN
13 Videos
- PERIODIC PROPERTIESBOOK - VK JAISWALCHAPTER - PERIODIC PROPERTIESEXERCISE - SUBJECTIVE PROBLEMS
22 Videos
- p-BLOCK ELEMENTSBOOK - VK JAISWALCHAPTER - p-BLOCK ELEMENTSEXERCISE - SUBJECTIVE PROBLEMS
36 Videos
- QUALITATIVE INORGANIC ANALYSISBOOK - VK JAISWALCHAPTER - QUALITATIVE INORGANIC ANALYSISEXERCISE - SUBJECTIVE PROBLEMS
5 Videos
Similar Questions
A compound contains three elements A,B and C, if the oxidation number of A=+2B=+5 and C=−2 then possible formula of the compound is
View Solution
View SolutionThe number of electrons for Zn2+ cation that have the value of azimuthal quantum number =0 is:
View SolutionIn phosphorus acid, If X is number of non-bonding electron pairs. Y is number of σ-bonds and Z is number of π-bonds. Then calculate value of "YxZ-X".
View SolutionConsider the following reaction
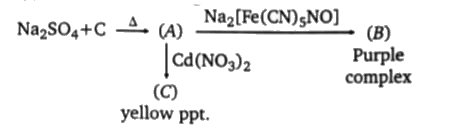
Then calculate value of ∣∣X2−Y2∣∣ (where X and Y are total number of electrons present t_(2g) and e_(g) orbitals respectively in d-block metal ion of compound B).
View SolutionELECTRONIC CONFIGURATION+ATOMIC RADII+IONIZATION ENTHALPY
View SolutionELECTRONIC CONFIGURATION+ATOMIC RADII+IONIZATION ENTHALPY
View SolutionELECTRONIC CONFIGURATION+ATOMIC RADII+IONIZATION ENTHALPY+ELECTRONEGATIVITY
View SolutionIonization enthalpy of an atom is equal to
View SolutionThe first ionization enthalpy of carbon atom is greater than that of boron whereas the reverse it true for the second ionization enthalpy Explain.
View SolutionWhich of the following atom has the lowest ionization enthalpy?
View SolutionThe ionization enthalpy of He+ ion is 19.60×10−18Jatom−1. The ionization enthalpy of Li2+ ion will be
View SolutionThe ionization enthalpy of He^(+) ion is 19.60 xx 10^(-18) J "atom"^(-1) The ionization enthalpy of Li^(+2) ion will be.
View SolutionCompared to first ionization enthalpy of an atom, the second is :
View SolutionCompared to first ionization enthalpy of an atom, the second is :
View SolutionCompared to first ionization enthalpy of an atom, the second is :
View Solution

