The incorrect statement regarding molecular orbital (s) is :
If there is a nodal plane perpendicular to the intermoleculear axis and lying between the nuclei of bonded atoms then corresponding orbitals is antibonding M.O.
If a nodal plane lies in the inter-nuclear axis, then corresponing orbitals is (pi) bonding M.O.
The σ-bonding molecular orbital does not contains the internuclear axis
The δ-bonding molecular orbitals possesses three nodal planes containing the internuclear axis
The correct Answer is:D
Topper's Solved these Questions
- CHEMICAL BONDING (ADVANCED)Book:VK JAISWALChapter:CHEMICAL BONDING (ADVANCED)Exercise:Level 3
Explore 92 Videos
- CHEMICAL BONDING (ADVANCED)Book:VK JAISWALChapter:CHEMICAL BONDING (ADVANCED)Exercise:ONE OR MORE ANSWER IS/ARE CORRECT
Explore 84 Videos
- CHEMICAL BONDING (ADVANCED)Book:VK JAISWALChapter:CHEMICAL BONDING (ADVANCED)Exercise:SUBJECTIVE PROBLEMS
Explore 72 Videos
- CHEMICAL BONDING (BASIC)Book:VK JAISWALChapter:CHEMICAL BONDING (BASIC)Exercise:Level 3 (Passive 11)
Explore 6 Videos
Similar Questions
Knowledge Check
The incorrect statement regarding cellulose is
Ait is a polymer of D-glucoseBIt has β−1,4− glycosidic linkageCit is used for making rayon fibreDit can be obtained by polymerisation of D-glucose in the lab.The incorrect statement regarding cellulose is -
AIt is a polymer of D-glucoseBIt has β -1,4-glucosidic linkageCIt is used for making rayon fibreDIt can be obtained by polymerization of D-glucoseThe incorrect statement regarding condensation is
AThe enolate is favoured at equilibriumBIt accomplishes the formation of a new C−C bondCThe key step in the mechanism of base cataysed reaction is attack of α-carbon of an enolate ion on the carbonyl carbonDDehydration of aldol gives α,β- unsaturated carbonyl compound
Incorrect statement regarding AIDS is
View SolutionAccording to MOT (Molecular Orbital Theory), the molecular orbitals are formed by mixing of atomic orbitals through LCAO (linear combination of atomic orbitals). The correct statement(s) about molecular orbitals is/are
Statement (A) : Bonding molecular orbitals are formed by addition of wave-functions of atomic orbitals of same phase
Statement (B) : Anti-bonding molecular orbitals are formed by subtraction of wave-functions of atomic orbitals of same phase
Statement (C) : Non-bonding molecular orbitals do not take part in bond formation because they belong to inner shells
Statement (D) : Anti-bonding molecular orbitals provide stability to molecules while bonding molecular orbitals make the molecules unstable.
View SolutionThe incorrect statement regarding structure of ozone
View Solution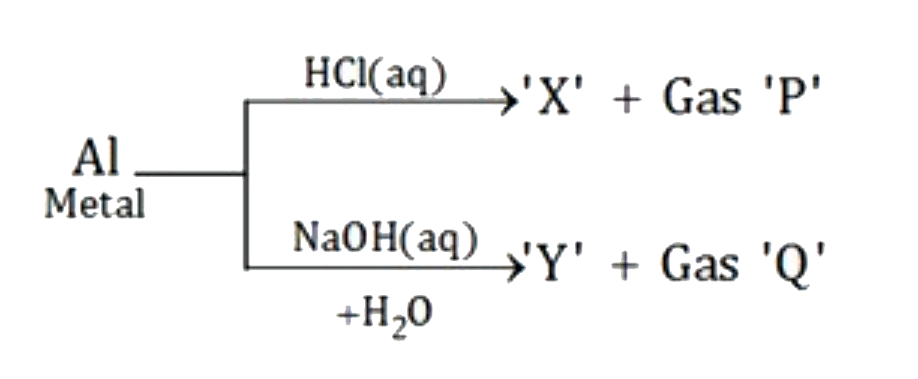
The incorrect statement regarding above reactions is
View SolutionThe correct statement regarding ClO−n molecular ion is:
View Solution

