Which of the following is a representative element ?
Zn
Sr
Cu
Fe
The correct Answer is:D
To determine which of the given options is a representative element, we need to understand the classification of elements in the periodic table.
1. Understand the Periodic Table Structure:
- The periodic table is organized into groups (vertical columns) and periods (horizontal rows).
- Groups contain elements with similar chemical properties.
2. Identify Representative Elements:
- Representative elements are found in the S and P blocks of the periodic table.
- The S block includes Group 1 (alkali metals) and Group 2 (alkaline earth metals).
- The P block includes Groups 13 to 18.
3. Identify Transition Metals:
- Transition metals are located in the D block, which corresponds to Groups 3 to 12 of the periodic table.
4. Analyze the Given Options:
- Look at the options provided in the question.
- Identify which of these options falls under the S or P block (representative elements) and which fall under the D block (transition metals).
5. Select the Representative Element:
- For example, if the options include elements like Zinc, Strontium, Copper, and Iron:
- Zinc is a transition metal (D block).
- Strontium is an alkaline earth metal (Group 2, S block).
- Copper is a transition metal (D block).
- Iron is a transition metal (D block).
- From this analysis, Strontium is the representative element.
Conclusion:
The representative element among the options is Strontium.
---
- The periodic table is organized into groups (vertical columns) and periods (horizontal rows).
- Groups contain elements with similar chemical properties.
- Representative elements are found in the S and P blocks of the periodic table.
- The S block includes Group 1 (alkali metals) and Group 2 (alkaline earth metals).
- The P block includes Groups 13 to 18.
- Transition metals are located in the D block, which corresponds to Groups 3 to 12 of the periodic table.
- Look at the options provided in the question.
- Identify which of these options falls under the S or P block (representative elements) and which fall under the D block (transition metals).
- For example, if the options include elements like Zinc, Strontium, Copper, and Iron:
- Zinc is a transition metal (D block).
- Strontium is an alkaline earth metal (Group 2, S block).
- Copper is a transition metal (D block).
- Iron is a transition metal (D block).
- From this analysis, Strontium is the representative element.
The representative element among the options is Strontium.
Similar Questions
Knowledge Check
Which of the following is not representative element?
ATelluriumBTantalumCThalliumDAstatineWhich of the following is not a representative element
ASodiumBBoronCCalciumDChromiumWhich of the following is not a representative element ?
AZ=37BZ=31CZ=54DZ=24
Whichone of the following is not the representative element ?
View SolutionIonization energies of five elements in kcal/mol are given below :
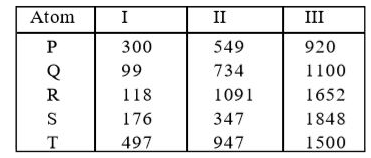
Which of the following pair represents elements of same group ?
View SolutionIonization energies of five elements in kcal/mol are given below:
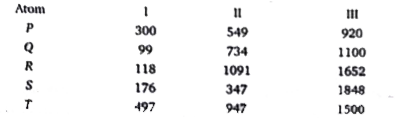
Q. Which of the following pair represents elements of same group?
View SolutionIonisation energies three hypothetical elements are given below (in kJ/mole) :
IIIIIIX1223401890Y999311100Z118122016552
Which of the following pairs represents elements could be of the same group?
View SolutionWhich of the following represents an element ?
View Solution

