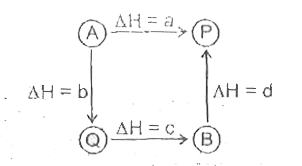
On the basis of Hess's law of constant heat summation, choose the correct relation:
A
b=a−cd+c
B
c=a−b+d
C
d=a+b+c
D
a−c=b+d
Video Solution
Text Solution
The correct Answer is:D
|
Answer
Step by step video, text & image solution for On the basis of Hess's law of constant heat summation, choose the correct relation: by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
Hess's law of constant heat summation is bases on
AE=mc2BE=hvCFirst law of thermodynamicsDConservation of massHess's law of constant heat summation is bases on
AE=mc2BConservation of massCFirst law of thermodynamicsDNone of the aboveHess's law of constant heat summationk is applied to calculate
Athe enthalpy of a reaction which is not feasibleBthe enthalpy of a reaction which does not go to completionCthe calforific value of a fuelDAll of these

