A system Xundergoes following changes
XP1V1T1→WP2V2T1→ZP3V2T2→XP1V1T1
The overall process may be called as
Reversible process
Cyclic process
Cyclic reversible process
Isochoric process
The correct Answer is:B
To determine the type of process described in the question, let's analyze the changes step by step.
Step 1: Identify the Initial and Final States
The system starts at state X with conditions P1,V1,T1. It then undergoes a series of changes to reach back to state X.
Step 2: Analyze Each Transition
1. From X to W: The system transitions from P1,V1,T1 to P2,V2,T1. Here, the pressure changes from P1 to P2 while the temperature remains constant at T1.
2. From W to Z: The system then transitions from P2,V2,T1 to P3,V2,T2. In this step, the pressure changes from P2 to P3 and the temperature changes from T1 to T2 while the volume remains constant at V2.
3. From Z back to X: Finally, the system returns from P3,V2,T2 back to P1,V1,T1. This means that the system undergoes changes in pressure, volume, and temperature to return to its original state.
Step 3: Determine the Type of Process
Since the system returns to its original state after undergoing a series of changes, this process is classified as a cyclic process. In a cyclic process, the initial and final states are the same, regardless of the path taken to get there.
Conclusion
The overall process described in the question is a cyclic process.
---
The system starts at state X with conditions P1,V1,T1. It then undergoes a series of changes to reach back to state X.
1. From X to W: The system transitions from P1,V1,T1 to P2,V2,T1. Here, the pressure changes from P1 to P2 while the temperature remains constant at T1.
2. From W to Z: The system then transitions from P2,V2,T1 to P3,V2,T2. In this step, the pressure changes from P2 to P3 and the temperature changes from T1 to T2 while the volume remains constant at V2.
Since the system returns to its original state after undergoing a series of changes, this process is classified as a cyclic process. In a cyclic process, the initial and final states are the same, regardless of the path taken to get there.
The overall process described in the question is a cyclic process.
Similar Questions
Knowledge Check
A gaseous system changes from state A(P1,V1,T1) to B(P2,V2,T2), B to C(P3,V3,T3) and finally from C to A. The whole process may be called
AReversible processBCyclic processCIsobaric processDSpontaneous process.A gaseous system changes from state A(P1,V1,T1) to B(P2,V2,T2) to C(P3,V3,T3) and finally from C to A. The whole process may be called :
AReversible processBCyclic processCIsobaric processDSpontaneous processA gaseous system changes from state A(P1,V1,T1) to B(P2,V2,T2), B to C (P3,V3T3) and finally from C to A. The whole process may be called
ACyclic processBReversible processCIsobaric processDSpontaneous process
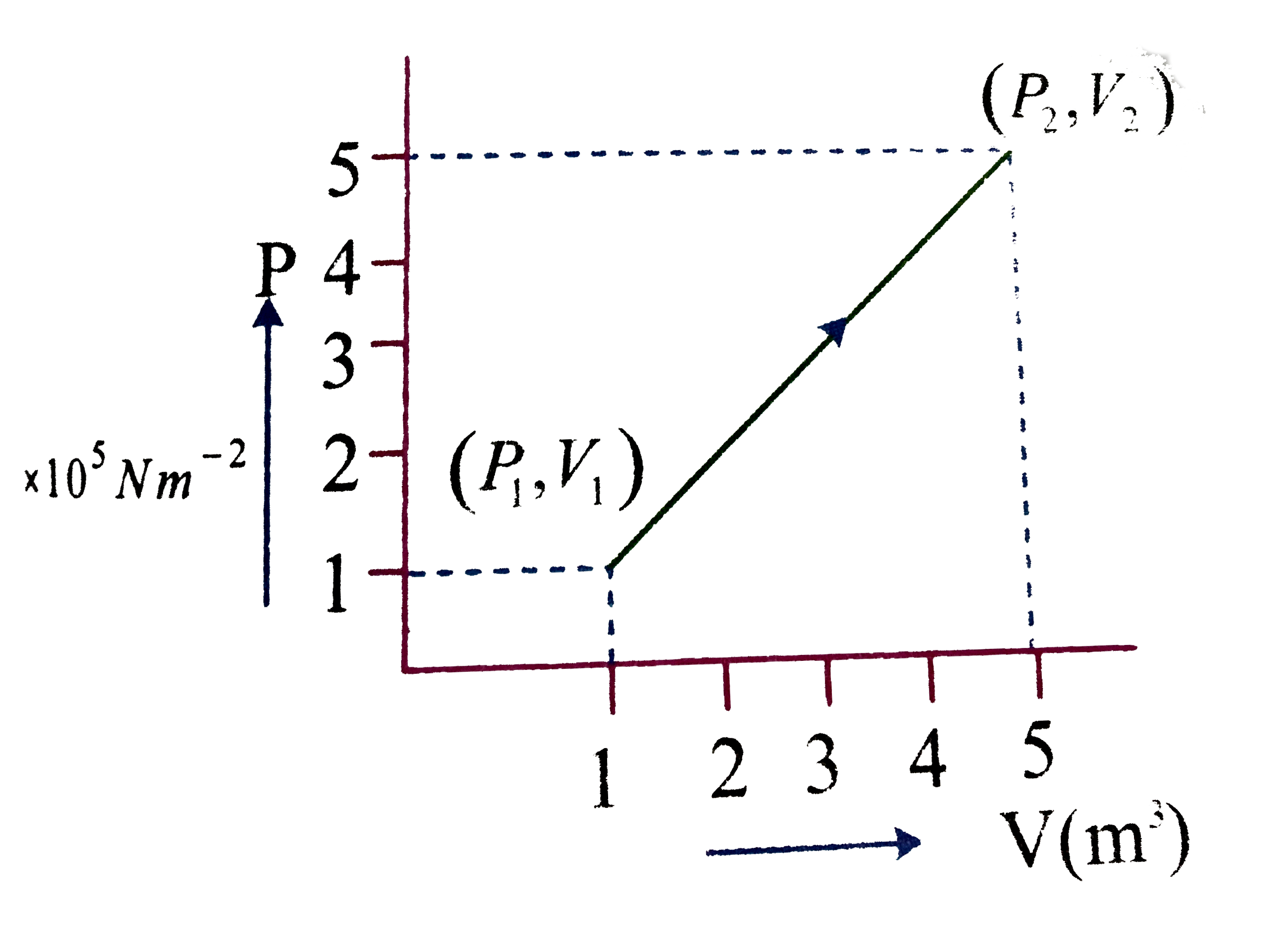
A system changes from the state (P1,V1) to (P2V2) as shwon in the diagram. The workdone by the system is
View SolutionAn ideal gasa undergoes through folowing cyclic procees.
1-2 : Reversible adialbatic comparession from
P1V1T1toP2V2T2
2-3 : Reversible isochoric heating from
P2V2T2 to P3V3T3
3-4 Reversible adialbatic expansion from
P3V3T3 to P4V4T4
4-1 Reversible isochoric cooling from
P4V4T4 to P1V1T1
Eefficiencyof the cycle is :
View SolutionAn ideal gas is expand from (p1,V1,T1) to (p2,V2,T2) under different conditions. The correct statement(s) among the following is (are):
View SolutionAn ideal gas is expand from (p1,V1,T1) to (p2,V2,T2) under different conditions. The correct statement(s) among the following is (are):
View SolutionAn ideal gas is expand from (p1,V1,T1) to (p2,V2,T2) under different conditions. The correct statement(s) among the following is (are):
View Solution

