The rate equation for a first order reaction is given by [R]=[R]0e−kt. A straight line with positive slope is obained by plotting [R]0= initial concentration of reactant, [R] = concentration of reactant at time, t Solution in Telugu
log [R]0[R]
[R] vs time
log [R] vs time
log [R][R]0 vs time
The correct Answer is:A
Step by step video, text & image solution for The rate equation for a first order reaction is given by [R] = [R]_(0) e^(-kt). A straight line with positive slope is obained by plotting [R]_(0)= initial concentration of reactant, [R] = concentration of reactant at time, t by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
C_(o) =initial concentration of the reactant C_(t) = concentration of the reactant at time t, k=rate constant of the reaction. Then the equation applicable for a first order reaction is
View SolutionThe slope in the plot of ln[R] vs. time gives (where [R] is the final concentration of reactant.)
View Solutionनिम्नलिखित ग्राफ दर्शाता है कि किस प्रकार अभिकारक R की t1/2 (अर्द्ध-आयु) प्रारंभिक अभिकारक सांद्रता a0 के साथ परिवर्तित होती है। अभिक्रिया की कोटि होगी
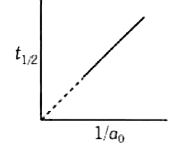
View Solutionअभिक्रिया A→B के लिये दर समीकरण r=k[A]0 है। यदि अभिकारक की प्रारंभिक सांद्रता a mol dm−3 है तब अभिक्रिया का अर्द्ध-आयुकाल होगा
View SolutionHow many time is required to complete reaction when initial concentration of reactant is [R]0 zero order reaction?
View SolutionThe slope of the straight line obtained by plotting rate versus concentration of reactant for a first order reaction is
View SolutionFor a given reaction the logarithm of the concentration of the reactant plotted against time gave a straight line with negative slope . The order of the reaction is
View Solution
