Which one of the four quantities does not indicate the thermodynamic state of a substance-volume, temperature, pressure and work?Solution in Bengali
work
Similar Questions
A quantity of gas occupies an initial volume V_(0) at pressure p_(0) and temperature T_(0) . It expands to a volume V (a) constant temperature and (b) constant pressure. In which case does the gas do more work ?
View SolutionA thermodynamics state function is a quantity
View Solutionरुद्धोष्म परिवर्तन के समय किसी ऊष्मागतिकी निकाय के निम्न गुणों में से कौन - सा गुण अपरिवर्तित रहता है - ताप , आयतन , दाब , और ऊष्मा की मात्रा |
View SolutionA thermodynamics state function is a quantity-
View SolutionWhich one of the following thermodynamic quantities is not a state function?
View SolutionOut of the parameters : temperature, pressure, work and volume, which parameter does not characterise the thermodynamics state of matter ?
View SolutionOut of the parameters : temperature, pressure, work and volume, which parameter does not characterise the thermodynamics state of matter ?
View Solutionचर राशियों ताप , दाब P, आयतन V तथा कार्य W में से किस चर युग्म द्वारा पदार्थ की ऊष्मागतिक अवस्था निर्देशित होती है,
View SolutionChoose the correct answer. A thermodynamic state function is a quantity. (a) used to determine heat changes. (b) whose value is independent of path. (c) used to determine pressure volume work. (d) whose value depends on temperature only.
View SolutionThe pair of parameters temperature T, pressure P. volume V and work W characterises the thermodynamic state of matter
View SolutionThe variables which determines the thermodynamic behaviour of a system are called thermodynamic variables.
Pick out the one which is NOT a thermodynamic variable.(temparature, pressure, work, volume)
View SolutionIf in a thermodynamical process, in a thermodynamical pressure and volume are equal to the final pressure and ,volume respectively, then
View SolutionA quantity of gas occupies an initial volume V0 at pressure p0 and temperature T0. It expands to a volume V (a) constant temperature and (b) constant pressure. In which case does the gas do more work ?
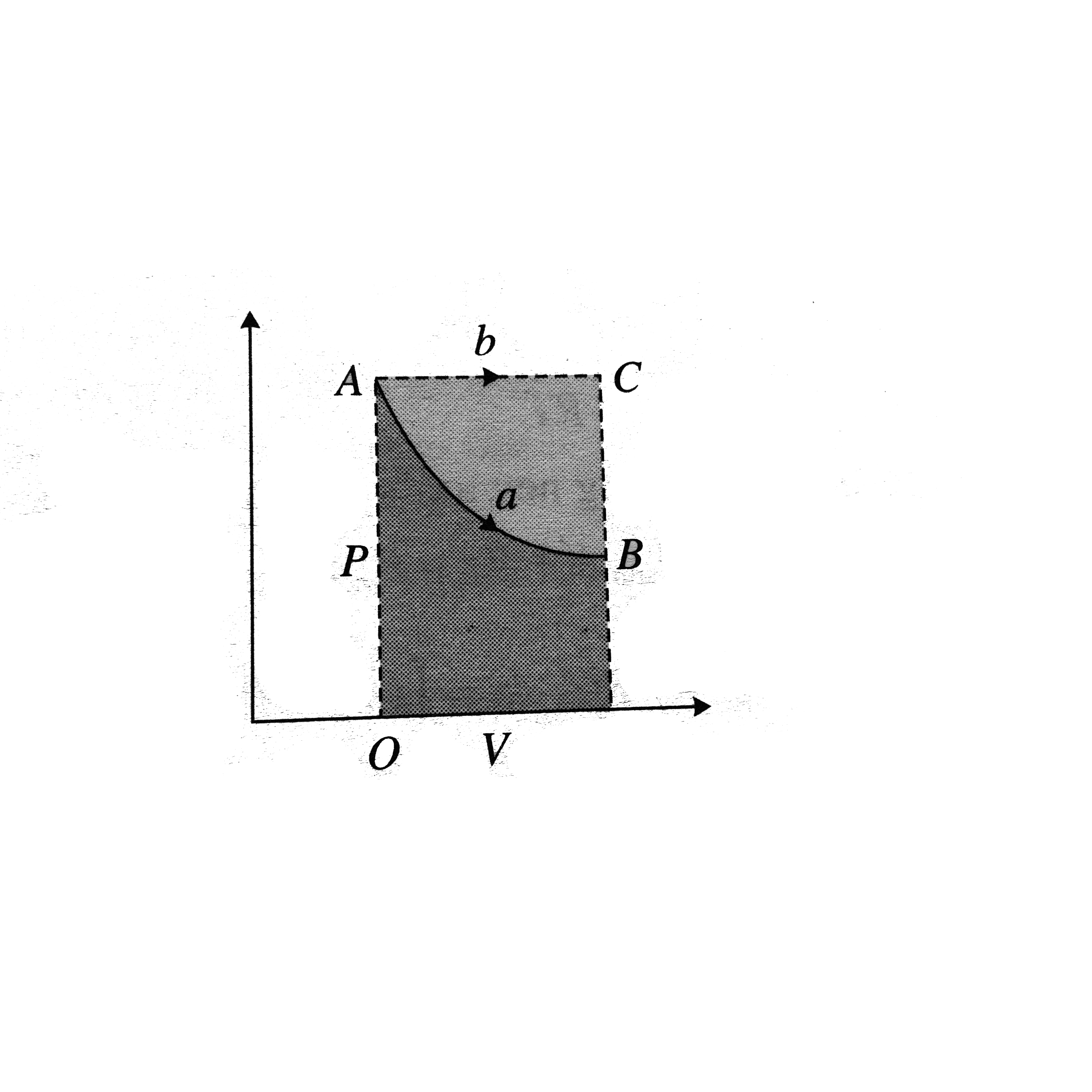
View SolutionWhich of the following quantities does not indicate any thermodynamic state of a substance?
View SolutionA thermodynamic state function is a quantity.
View Solution
