In which process does the heat accepted or rejected become zero?Solution in Bengali
adiabatic
Similar Questions
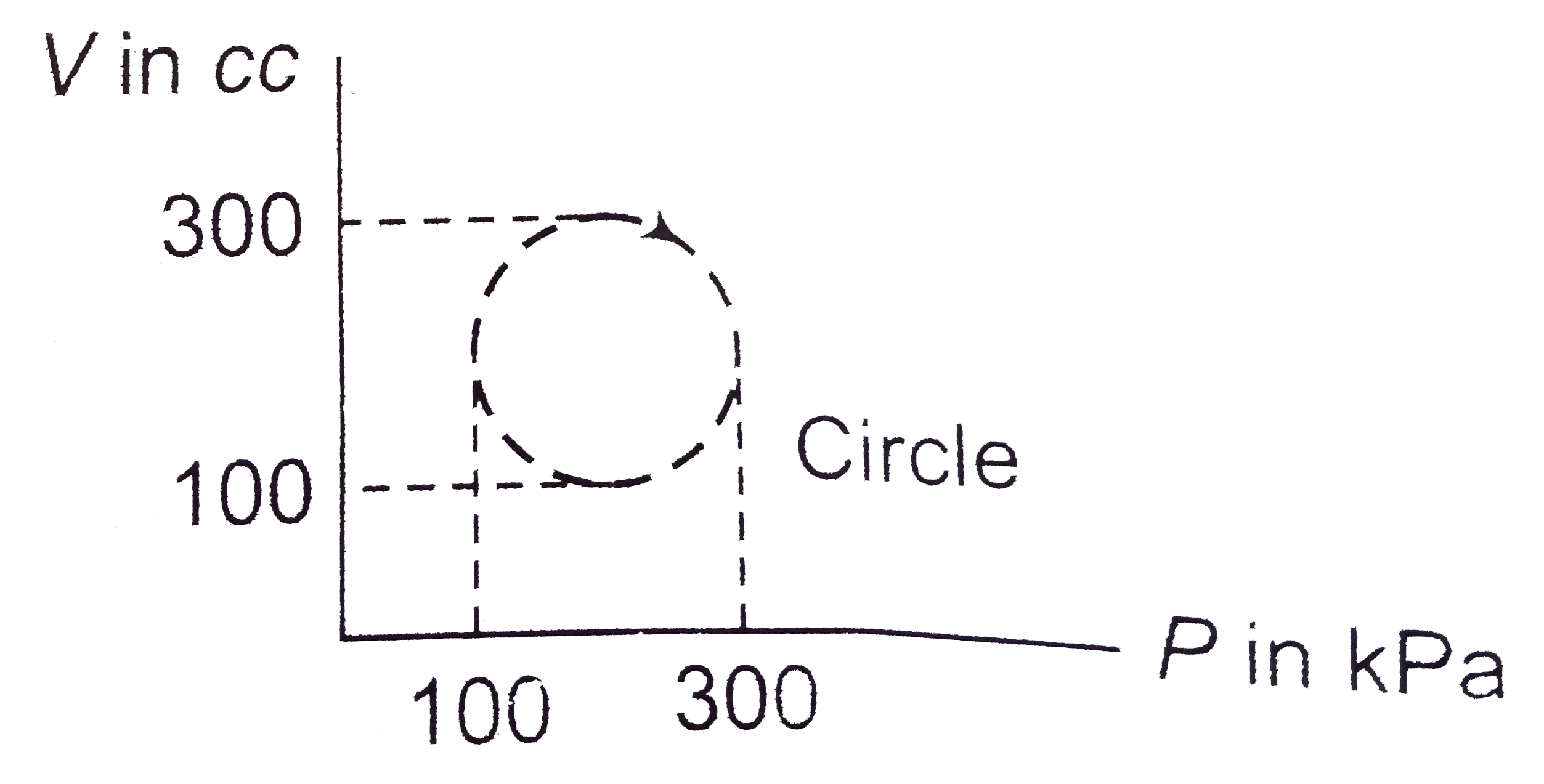
Calculate the heat rejected by a system in going through the cyclic process shown in figure.
View SolutionHeat engine rejects some heat to the sink. This heat
View SolutionA sample of oxygen is heated in a process for which the molar specific heat capacity is 2R. During the process the temperature becomes (32)1/3 times of the original temperature. How does the volume of the gas change?
View SolutionWhich of the following metallurgical process does not involve heating?
View SolutionWhich of the following metallurgical process does not involve heating?
View SolutionThe theory of panagenesis was rejected due to the acceptance of :
View Solutionनिम्नलिखित में किस प्रक्रिया द्वारा धातु अधिक मजबूत हो जाती है?
View SolutionWhich of the following process does not involve heating?
View SolutionAn ideal gas is taken through a process in which the process equation is given as P=kV^(alpha) , where k and alpha are positive constants. Find the value of alpha for which in this process molar heat capacity becomes zero.
View SolutionAt which temperature does the kinetic energy of gas molecules become zero?
View Solutionজারণে মৌল ইলেকট্রন গ্রহণ করে না বর্জন করে?
View SolutionIf the system does not lose heat or does not receive heat, then the process is called ......... .
View Solutionनिम्न में से किस प्रक्रिया में ऊष्मा संवहन प्रयुक्त नहीं होता है
View SolutionHow does the amount of heat received or rejected by an object depend on the mass of the object?
View SolutionA Carnot engine receives 2.0 kj of heat from a reservoir at 500 K, does some work, and rejects some heat to a reservoir at 351 K. How much heat is rejected.
View Solution

