Which type of overlapping result in the formation of (i) sigma bond (ii) pi bond.Solution in Kannada
(i) Sigma bond-overlapping of orbital along the axis.
(ii) pi bond overlapping sideways of two p-orbital.
Similar Questions
Why does type of overlap given in the following figure not result in bond formation?
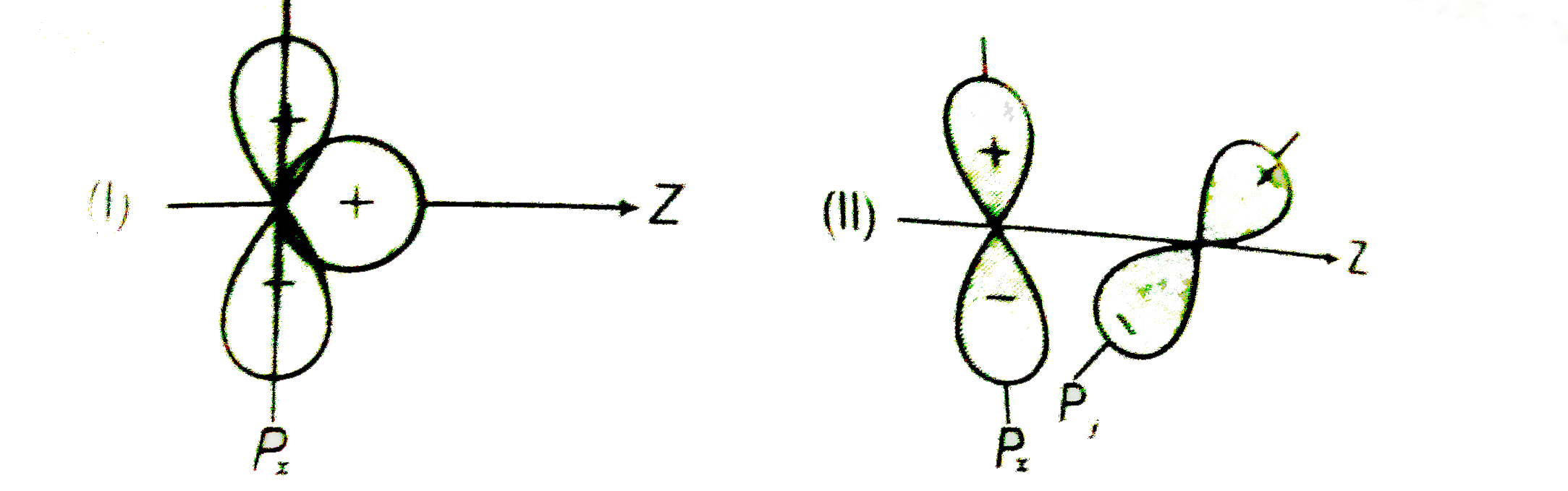
View SolutionWhy does type or overlap given in the following figure not result in bond formation ?
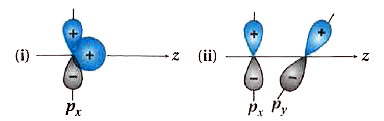
View SolutionWhich type of overlapping reusults in the formation of a π bond?
View SolutionValence Bond Theory || Concept OF Overlapping Axial Overlapping and Formation OF Sigma Bonds
View SolutionA covalent bond will be formed by the overlapping of atomic orbitals having single electron of opposite spin, according to the overlapping of atomic orbitals the covalent bond may be of two types :
(i) Sigma bond (σ) (ii) Pi bond (π)
Sigma bond is stronger bond than the Pi-bond. If atomic orbitals overlap about the nuclear axis then sigma bond is formed but when atomic orbitals overlap sideway then Pi-bond is formed.
Compound having maximum bond angle is :
View SolutionA covalent bond will be formed by the overlapping of atomic orbitals having single electron of opposite spin, according to the overlapping of atomic orbitals the covalent bond may be of two types :
(i) Sigma bond (σ) (ii) Pi bond (π)
Sigma bond is stronger bond than the Pi-bond. If atomic orbitals overlap about the nuclear axis then sigma bond is formed but when atomic orbitals overlap sideway then Pi-bond is formed.
The strength of bonds formed by 2s-2s,2p-2p and 2p-2s overlap has the order :
View SolutionA covalent bond will be formed by the overlapping of atomic orbitals having single electron of opposite spin, according to the overlapping of atomic orbitals the covalent bond may be of two types :
(i) Sigma bond (σ) (ii) Pi bond (π)
Sigma bond is stronger bond than the Pi-bond. If atomic orbitals overlap about the nuclear axis then sigma bond is formed but when atomic orbitals overlap sideway then Pi-bond is formed.
Compound having maximum bond angle is :
View SolutionA covalent bond will be formed by the overlapping of atomic orbitals having single electron of opposite spin, according to the overlapping of atomic orbitals the covalent bond may be of two types :
(i) Sigma bond (σ) (ii) Pi bond (π)
Sigma bond is stronger bond than the Pi-bond. If atomic orbitals overlap about the nuclear axis then sigma bond is formed but when atomic orbitals overlap sideway then Pi-bond is formed.
The strength of bonds formed by 2s-2s,2p-2p and 2p-2s overlap has the order :
View Solution
