Define Hydrogen Bond ? Explain its type with example.Solution in Kannada
Video Solution
Text Solution
Electrostatic force of attraction that exists between atom of one molecule electronegative atom of same of other molecules is called hydrogen bond.
Type of hydrogen bond :
i. Intermolecular hydrogen bond.
ii. Intermolecular hydrogen bond.
i. Intermolecular hydrogen bond : Eelectrostatic force of attraction that exists between hydrogen of one molecule and electronegative atom of another molecule is called intermolecular hydrogen atom.
1 H2O
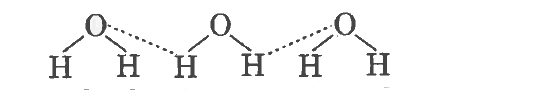
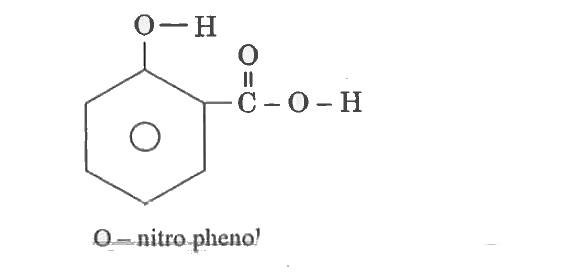
ii. Intermolecular Hydrogen bond : Electrostatic force of attraction that exists between hydrogen atom of one molecule electronegative atom of the same molecule.
Example : Salicylic acid C6H4COOH(OH)
|
Similar Questions
(a) Define hydrogen bond and it types.
View SolutionWhat is a hydrogen bond? Explain the different hydrogen bonds with examples.
View Solutionవిభిన్న హైడ్రోజన్ బంధాలను ఉదాహరణాలతో వివరించండి.
View SolutionDefine aestivation.
View SolutionWhat is hydrogen bond ? Explain its characteristics with example.
View SolutionExplain types of Hydrogcn bond example and its effect on physical properties. OR Give difference between intermolecular hydrogen bond and intramolecular hydrogen bond.
View SolutionDefine collision .Explain its types with examples.
View Solution
