Give reasons for the following :
(t) Ethyl iodide undergoes SN2 reaction faster than ethyl bromide.
(ii) (±) 2-Butanol is optically inactive.
(iii) C- X bond length in halobenzene is smaller than C-X bond length in CH3−X.
(i) Ethyl iodide I− ion is better leaving group than Br− ion, therefore, ethyl iodide reacts faster than ethyl bromide in SN2 reaction with OH− ion.
Similar Questions
Give reasons for the following:
(i) Ethyl iodide undergoes SN2 reaction faster than ethyl bromide.
(ii) (±) 2-Butanol is optically active.
(iii) C - X bond length in halobenzene is smaller than C - X bond length in CH3−X.
View SolutionGive reasons:
(i). C−Cl bond length in chlorobenzene is shorter than C−Cl bond length in CH3−Cl
(ii). The dipole moment of chlorobenzene is less than of cyclohexyl chloride.
(c). SN1 reactions are are accompanied by racemisation is optically active alkyl halides.
View Solution
Knowledge Check
The decreasing order of C-X bond length in CH3−X is
ACH2I>CH3Br>CH3Cl>CH3FBCH3F>CH3Cl>CH3Br>CH3ICCH3F>CH3Cl>CH3I>CH3BrDCH3I>CH3Cl>CH3F>CH3BrWhich of the following have larger C-O bond length than C-O bond length of
CH3−O∣∣C−CH3?A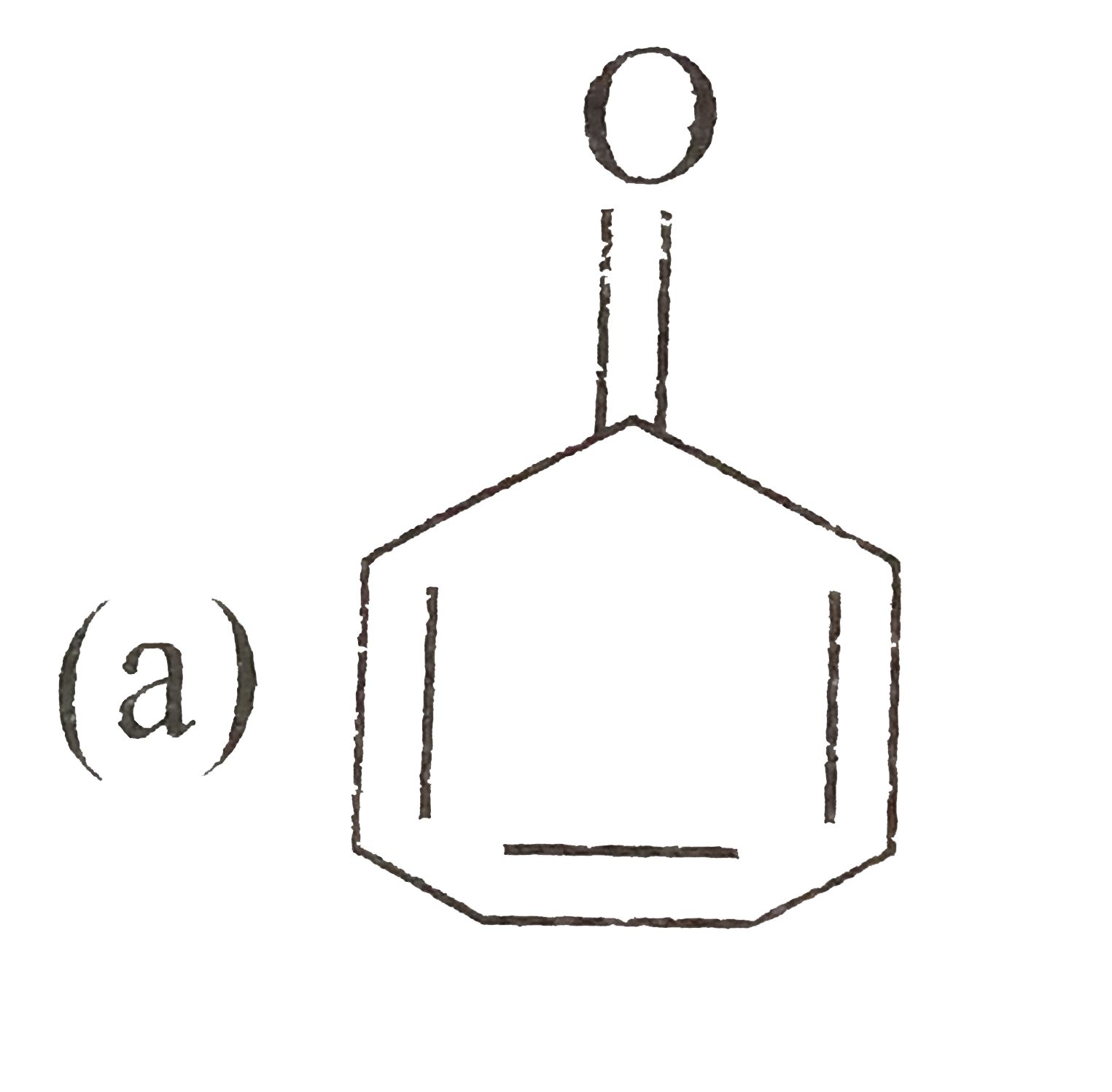 B
B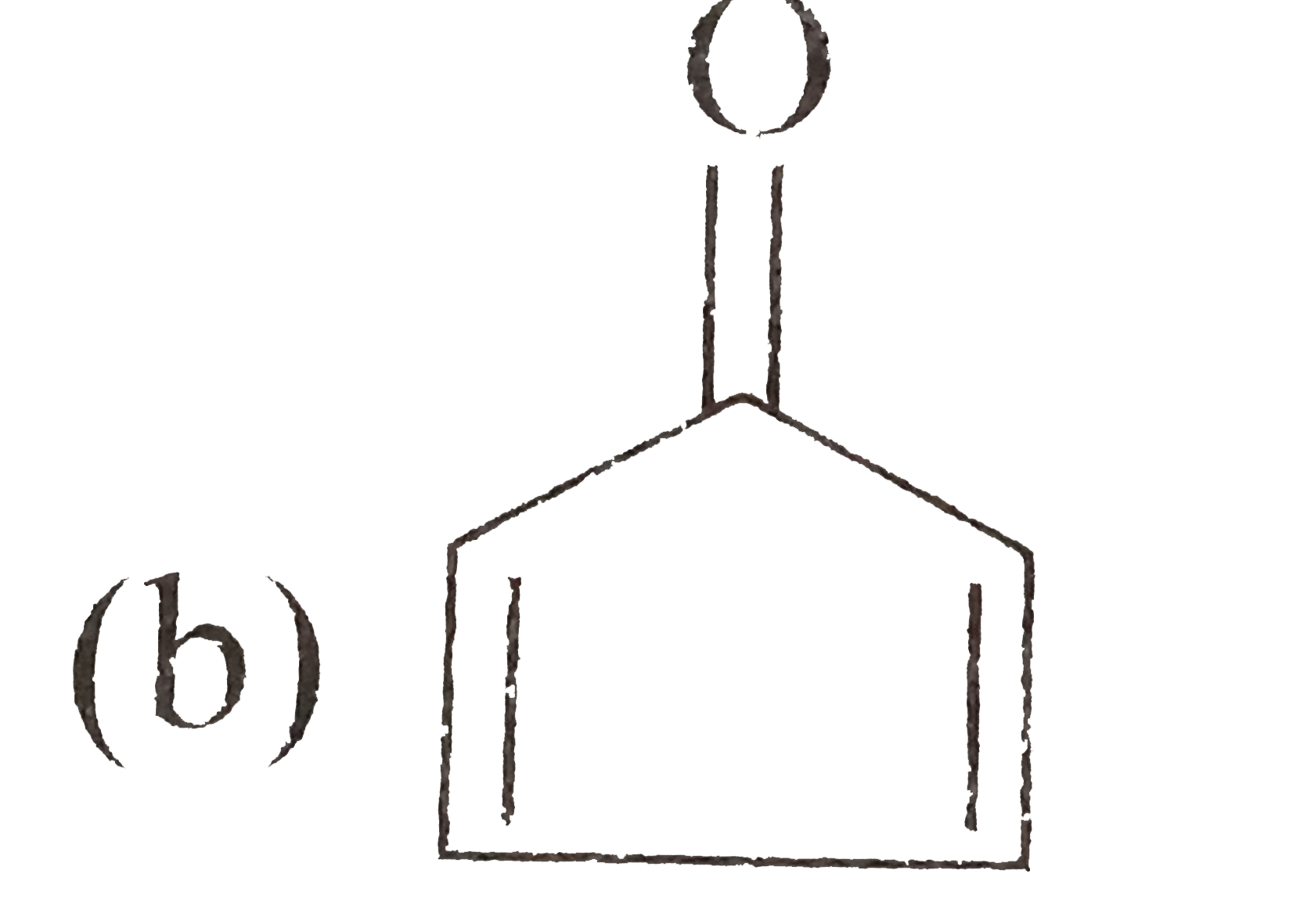 C
C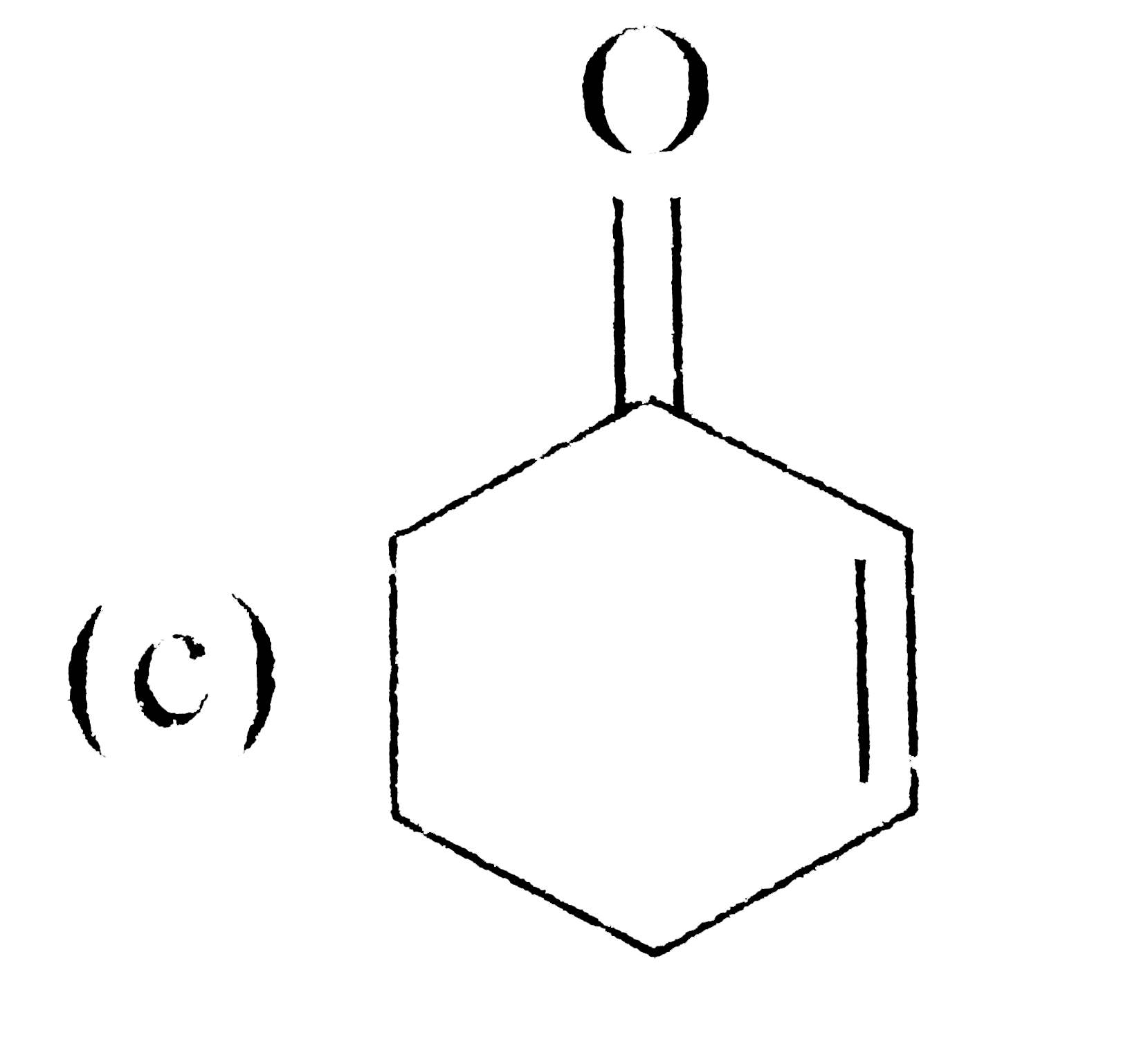 D
D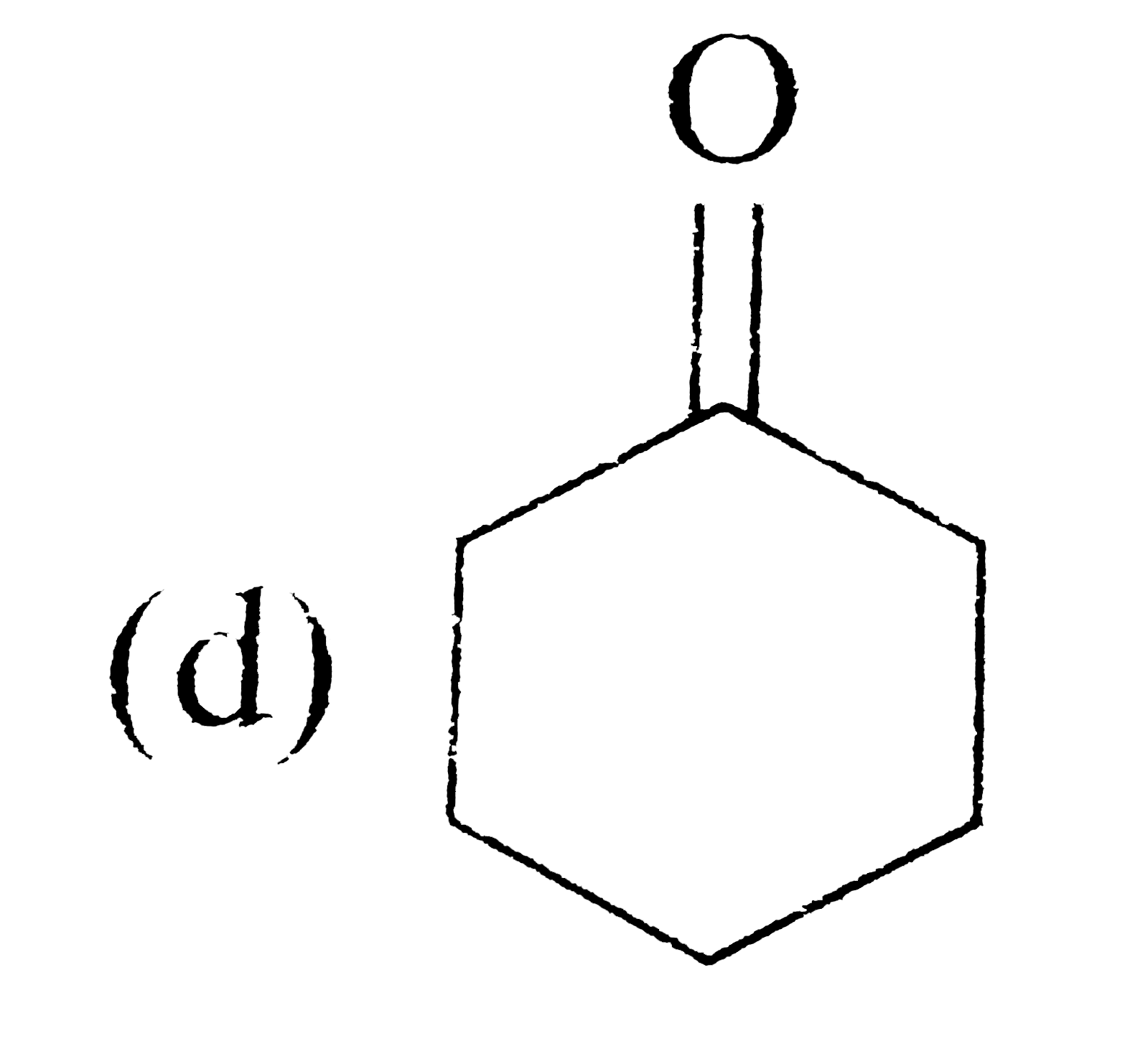
Assertion :- Bond energy of F2 is less than Cl2.
Reason :- Bond length of F2 is smaller than Cl2.AIf both Assertion & Reason are True & the Reason is a correct explanation of the Assertion.BIf both Assertion & Reason are True but Reason is not a correct explanation of the Assertion.CIf Assertion is True but the Reason is False.DIf both Assertion & Reason are False.
Give reasons :
C-Cl bond length in chlorobenzene is shorter than C -- Cl bond length in CH3−Cl.
View SolutionGive reasons for the following:
CH2=CHΘ is more basic than HC≡CΘ
View SolutionGive reasons for the following:
CH2=CHΘ is more basic than HC≡CΘ
View SolutionGive reasons for the following :
C−OH bond length in CH3OH is slightly more than C−OH bond length in phenol.
View SolutionAccount for the following :
(i) PCl5 is more covalent than PCl3.
(ii) Iron on reaction with HCl forms FeCl2 and not FeCl3.
(iii) The two O-O bond lengths in the ozone molecule are equal.
View Solution

