Give reasons for the following :
C−OH bond length in CH3OH is slightly more than C−OH bond length in phenol.
(i) Smaller than tetrahedral angle is due to repusion between the unshared or lone pairs of electrons on oxygen withi makes it smaller than 109∘−28..
(ii) C−OH bond length in CH3OH is slightly more than the C−OH bond length in phenol due to two reasons :
Carbon atom of the ring to which −OH is attached is sp2 hybridised while corresponding carbon in alcohol is sp3 hybridised. A sp2 hybridised orbital gives a smaller bond.
Topper's Solved these Questions
Similar Questions
Knowledge Check
C-O bond length in phenol is less than C-O bond length in methyl alcohol because
Apartial double bond character due to resonanceBpartial double bond character due to inductive effectCmore electronegativity of oxygenDoxygen contain two lone pair of electronsTwo -OH bonds are present in
AH4P2O5BH4P2O6CH3PO4DH4P2O7
Give reasons:
(i). C−Cl bond length in chlorobenzene is shorter than C−Cl bond length in CH3−Cl
(ii). The dipole moment of chlorobenzene is less than of cyclohexyl chloride.
(c). SN1 reactions are are accompanied by racemisation is optically active alkyl halides.
View SolutionGive reasons for the following :
(i) Phenol is more acidic than methanol.
(ii) The C-O-H bond angle in alcohols is slightly less than the tetrahedral angle (109∘28').
(iii) (CH3)3C−O−CH3 on reaction with HI gives (CH3)3C−IandCH3−OH as the main products and not (CH3)3C−OHandCH3−I.
View SolutionGive reasons for the following :
(i) Phenol is more acidic than methanol.
(ii) The C−O−H bond angle in alcohols is slightly less than the tetrahedral angle (109∘28.).
(iii) (CH)(3))2C−O−CH3 on reaction with HI gives (CH3)3C−IandCH3−OH as the main products and not (CH3)3C−OHandCH3−I.
View SolutionGive reasons:
(i). C−Cl bond length in chlorobenzene is shorter than C−Cl bond length in CH3−Cl
(ii). The dipole moment of chlorobenzene is less than of cyclohexyl chloride.
(c). SN1 reactions are are accompanied by racemisation is optically active alkyl halides.
View SolutionGive reasons :
C-Cl bond length in chlorobenzene is shorter than C -- Cl bond length in CH3−Cl.
View SolutionGive reasons for the following :
(t) Ethyl iodide undergoes SN2 reaction faster than ethyl bromide.
(ii) (±) 2-Butanol is optically inactive.
(iii) C- X bond length in halobenzene is smaller than C-X bond length in CH3−X.
View Solution


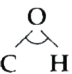 in alcohol is slightly less than the tetrahedral angle.
in alcohol is slightly less than the tetrahedral angle.