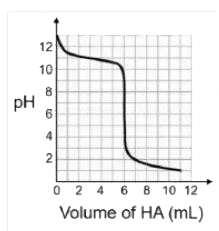
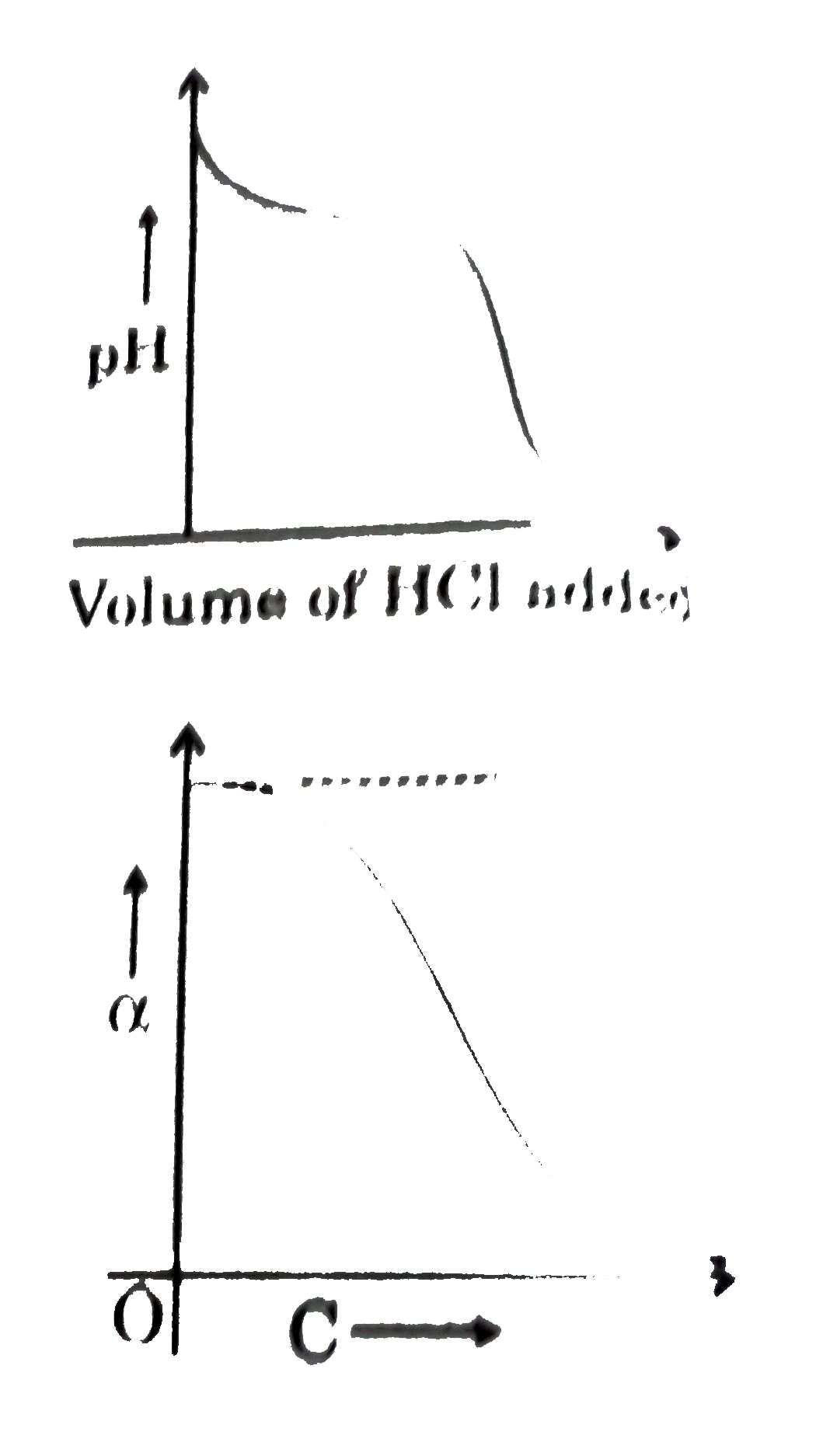
A solution of 0 .1 weak base (B )is titrated with 0.1 M of a strong acid ( HA) . The variation of pH of the solution will be the volume of HA added is shown in the figure below. What is the pKb of the base ? The neutralization reaction is given by B+HA→HA→BH++A−
The correct Answer is:03.00
At 3 ml pH of solution is 11
B+HA→BH++A−
at 3 ml best butter action is shown
at 3 ml best buffer action is shown
So POH=PKb=3 (As basic buffer is formed )
So PKb=3
Similar Questions
When 100 mL of 0.1 M NaCN solution is titrated with 0.1 M HCl solution the variation of pH of solution with volume of HCl added will be :
View SolutionA weak base, BOH is titrated with a strong acid HA. When 10 ml of HA is added, the pH of the solution is 10.2 and when 25 ml is added, the pH of the solution is 9.1. Calculate the volume of acid that would be required to reach equivalence point.
View Solution
Knowledge Check
A weak base (BOH) with Kb=10−5 is titrated with a strong acid (HCl), At 3/4 th of the equivalence point, pH of the solution is:
A5+log3B14−5−log3C14−5+log3D9.523.1.2 of a monprotic acid HA, is titrated with 0.222 M NaOH solution. The pH of the solution is monitrored with pH meter. A portion of the titration curve is shown in the diagram.
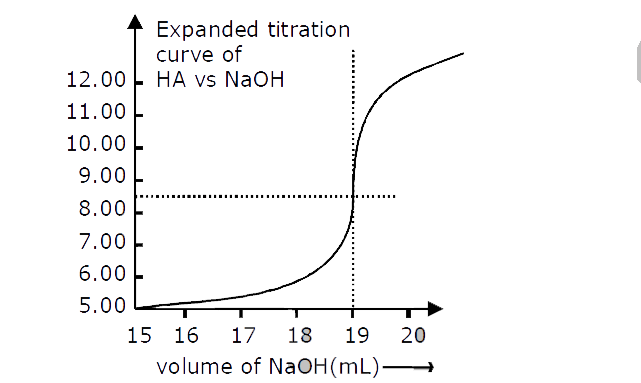
What is the molar mass of HA?A180B222C282DNone1.2 of a monprotic acid HA, is titrated with 0.222 M NaOH solution. The pH of the solution is monitrored with pH meter. A portion of the titration curve is shown in the diagram.
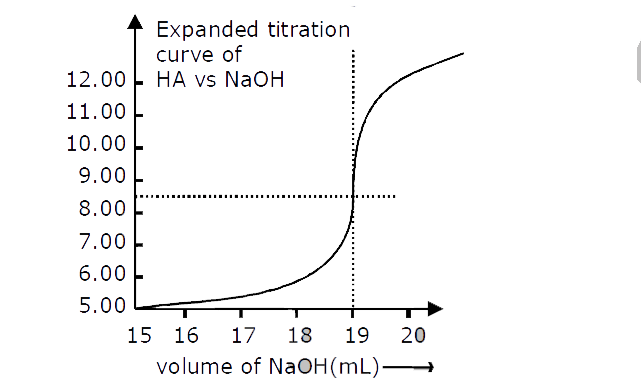
What is the pH of solution at the equivalence point?A3.50B7.00C8.40D5
A weak base BOH is titrated with strong acid HA. When 10mL of HA is added, the pH is 9.0 and when 25mL is added, pH is 8.0. The volume of acid required to reach the equivalence point is
View SolutionA solution of a substances is titrated against a strong base ( or acid ) ,volume V of strong base (or acid) is plotted against pH of the solution (as shown in figure) .The substances could be :
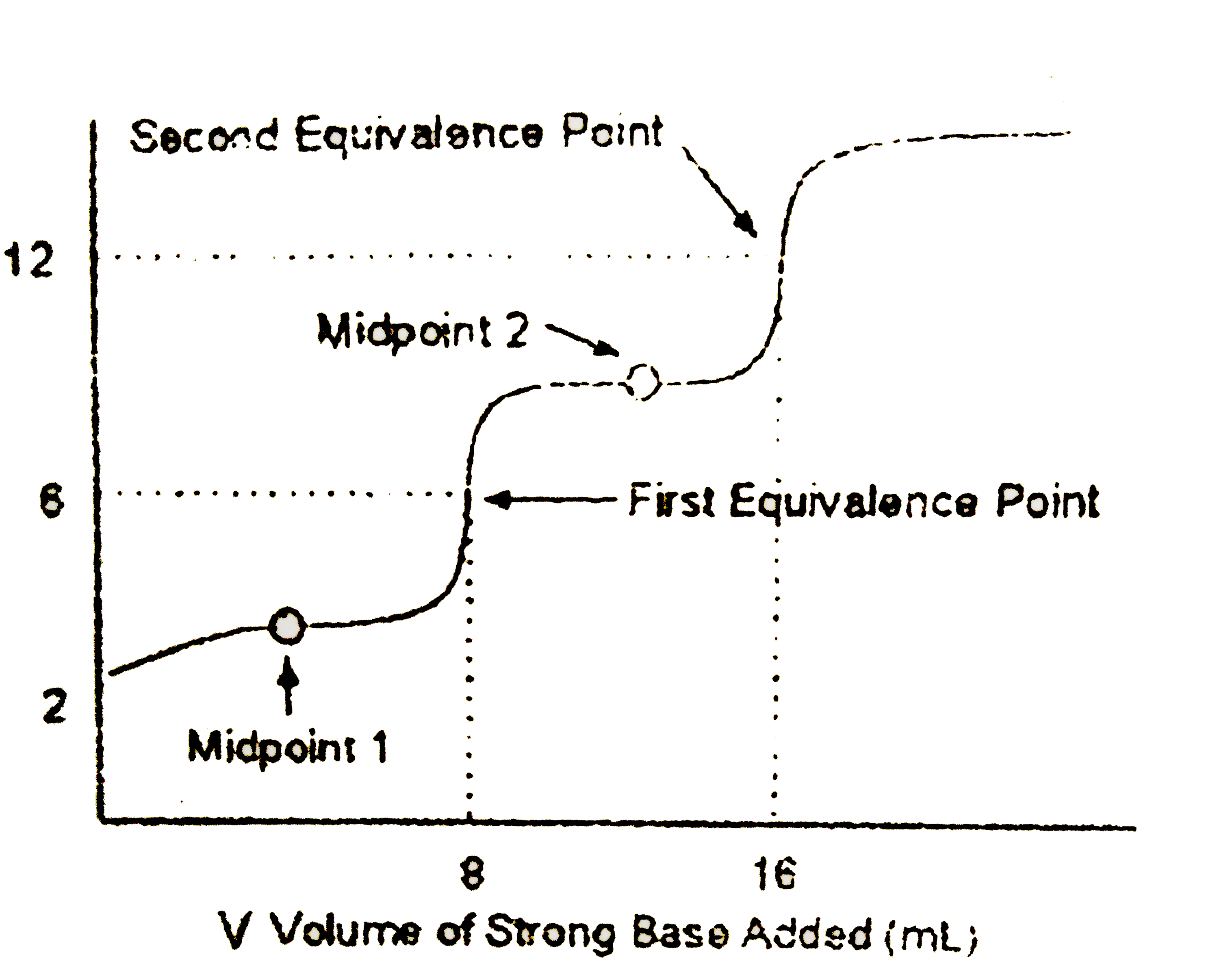
View Solution20.0 L of 0.2 M weak acid (pKa=5.0) is titrated against 0.2 M strong base. What is the pH at the equivalence point ?
View SolutionThe pH of a solution of weak base at neutralisation with strong acid is 8.Kb for the base is
View SolutionWhich is/are correct statement ?
(P) When 100 mL of 0.1MNaCN solution is titrated with 0.1MHCI solution the variation of pH of solution with volume added will be (as shown in fig.)
(Q) Variation of degree of dissociation α with concentration for a weak electrolyte at a particular temperature is best represented by (as shown in fig.)
(R) 0.1M acetic acid solution is titrated aganist 0.1MNaOH solution. the difference in pH between 1/4 and 3/4 stages of neutralization of acid will be 2 log 3.
View Solution
