A laboratory analysis of an organic compound gives the following mass percentage composition: C = 60%, H = 4.48% and remaining oxygen. Find out the Empirical Formula of the compound.Solution in Tamil
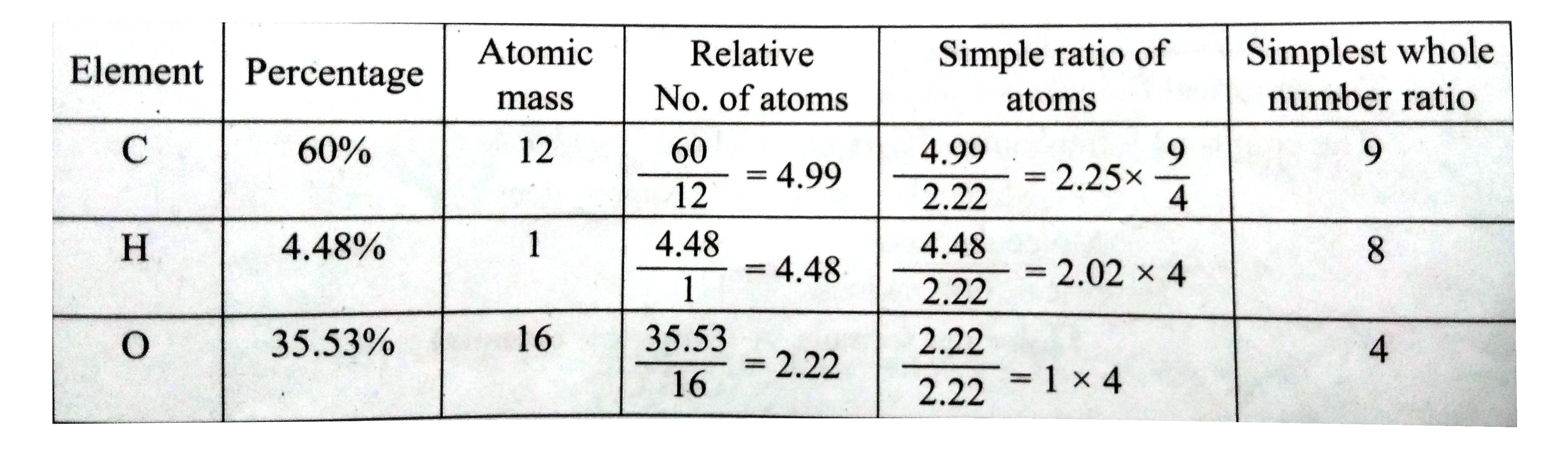
∴ The empirical formula is C9H8O4
Similar Questions
A compound on analysis gave the following percentage composition: C = 24.47%, H= 4.07 %, Cl = 71.65%. Find out its empirical formula.
View SolutionAn organic compound on analysis gave the following composition :
C = 57.8% H = 3.6%
and rest is oxygen. Its empirical formula is :
View SolutionAn organic compound on analysis gave the following percentage composition : C = 57.8%, H = 3.6% and the rest is oxygen. The vapour density of the compound was found to be 83. Find out the molecular formula of the compound
View SolutionAn organic compound on analysis gave the following results : C=54.5% ,O= 36.4 % H=9.1 % . The empirical formula of the compound is ____.
View SolutionThe percentage of C, H and Cl in an organic compounds is 10%, 0.84%, 89.2% respectively. Find out its empirical formula.
View SolutionAn organic compound with C =40 % and H= 6.7% will have the empirical formula
View SolutionA carbon compound on analysis gave the following percentage composition, carbon 14.5%, hydrogen 1.8%, chlorine 64.46%, oxygen 19.24%. Calculate the empirical formula of the compound.
View Solution
