What are intermolecular hydrogen bonds? Explain with example.Solution in Tamil
Video Solution
Text Solution
(i) Intramolecular hydrogen bonds are those which occur within one single molecule. This occurs between two functional groups within a molecule.
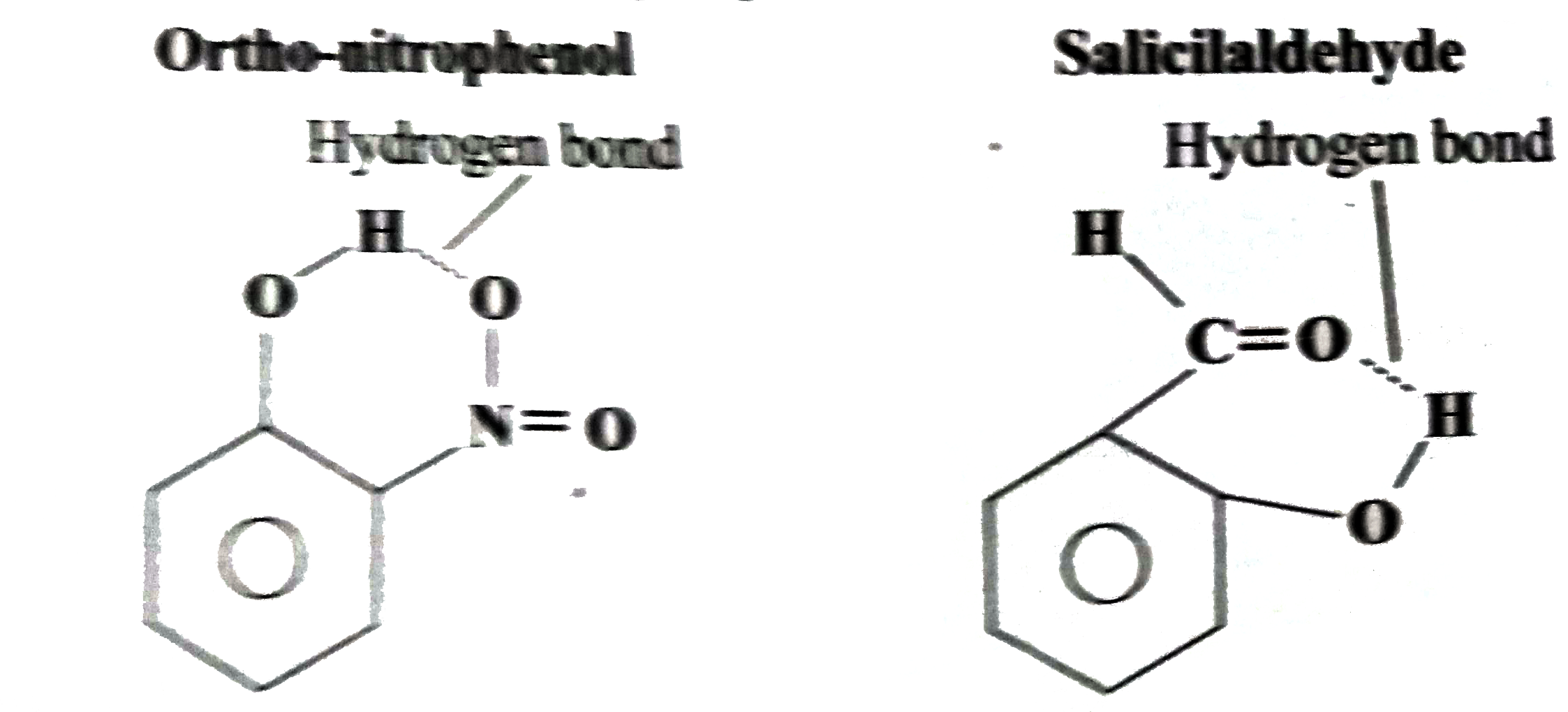
An intramolecular hydrogen bond (dashed lines) joins the OH group to the doubly bonded oxygen atom of the carboxyl group on the same molecule, e.g., Salicylic acid
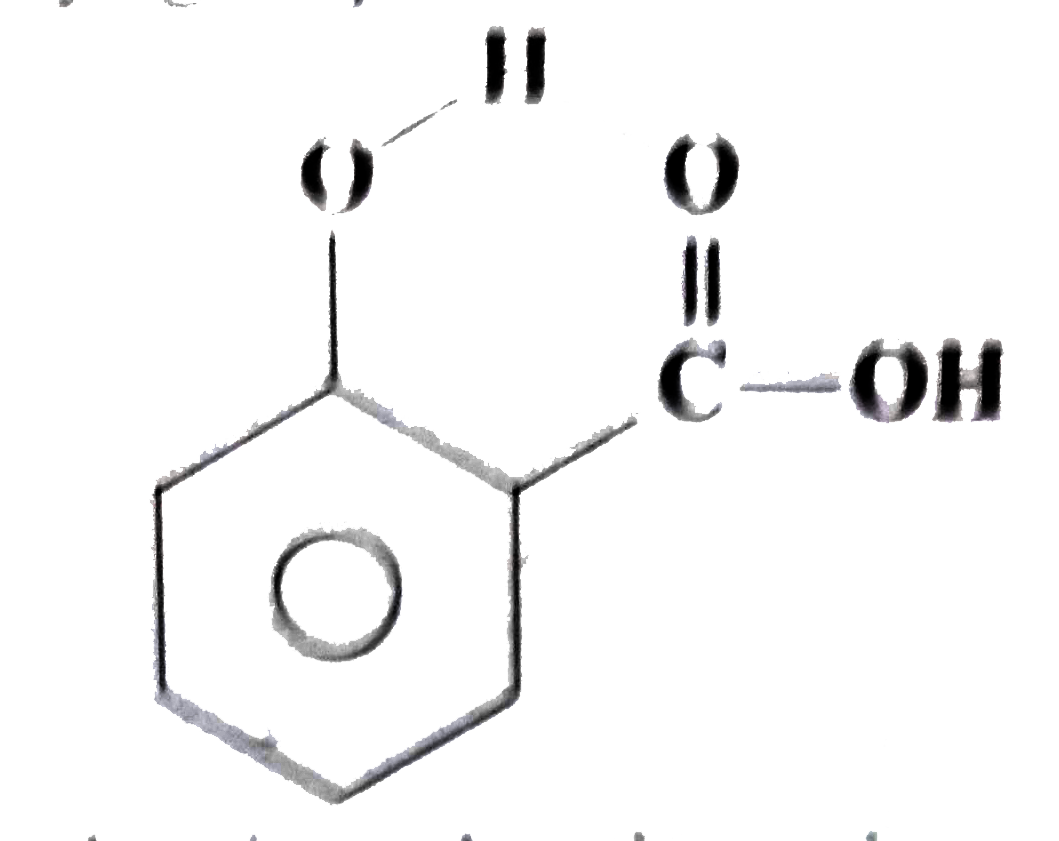
(iii) Salicylic acid act as an analgesic and antipyretic.
|
Similar Questions
Define hydrogen bond. Give an example for the molecule having (i)Intermolecular hydrogen (ii) intramolecular hydrogen bond.
View Solutionअन्तराआण्विक हाइड्रोजन बन्ध पाया जाता है
View SolutionWhat are intermolecular hydrogen bonds? Explain with example.
View SolutionWhat is a hydrogen bond? Explain the different hydrogen bonds with examples.
View SolutionIn solid hydrogen, the intermolecular bonding is
View SolutionWhat is hydrogen bond ? Explain its characteristics with example.
View SolutionExplain types of Hydrogcn bond example and its effect on physical properties. OR Give difference between intermolecular hydrogen bond and intramolecular hydrogen bond.
View Solution

