What are intermolecular hydrogen bonds? Explain with example.Solution in Tamil
Video Solution
Text Solution
(i) Intermolecular hydrogen bonds occur between two separate molecules. They can occur between any numbers of like or unlike molecules as long as hydrogen donors and acceptors are present in positions in which they can interact.
(ii) For e.g., Intermolecular hydrogen bonds can occur between ammonia molecules alone, between water molecules alone or between ammonia and water.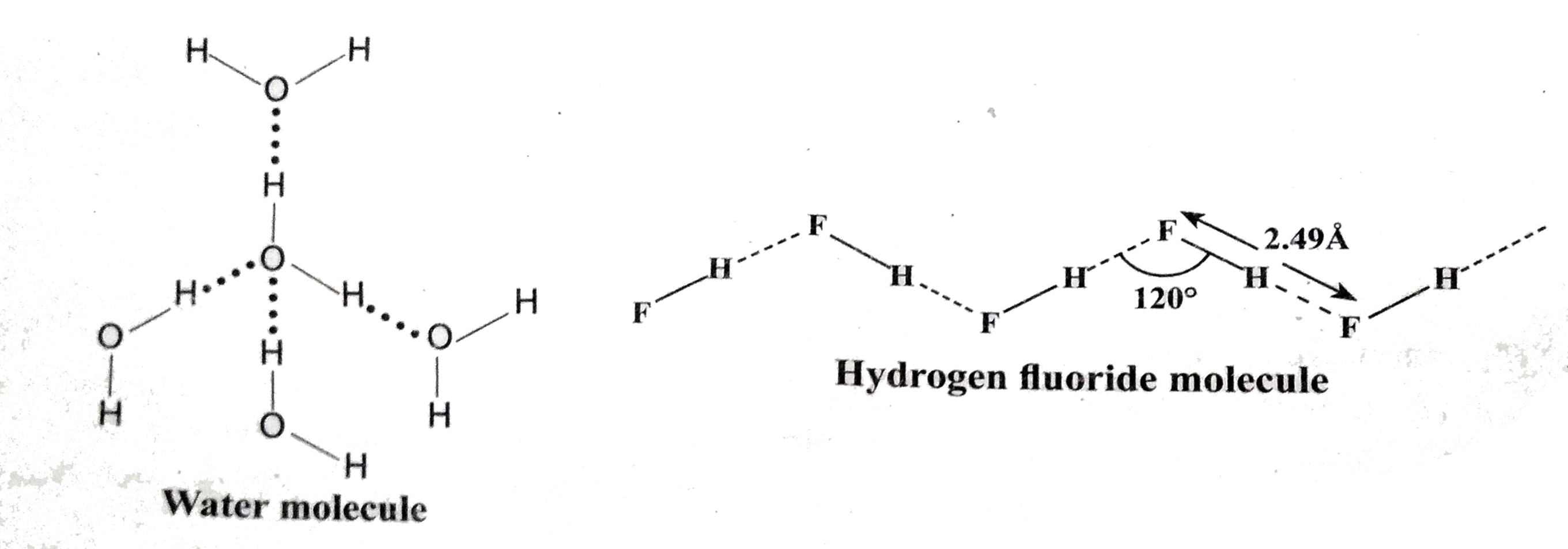
|
Similar Questions
अन्तराआण्विक हाइड्रोजन बन्ध पाया जाता है
View SolutionWhat are intermolecular hydrogen bonds? Explain with example.
View SolutionWhat is a hydrogen bond? Explain the different hydrogen bonds with examples.
View SolutionIn solid hydrogen, the intermolecular bonding is
View SolutionWhat is hydrogen bond ? Explain its characteristics with example.
View SolutionExplain types of Hydrogcn bond example and its effect on physical properties. OR Give difference between intermolecular hydrogen bond and intramolecular hydrogen bond.
View SolutionIntermolecular Hydrogen bonding is strongest in
View Solution

