Write short note an freezing point depression in freezing point and cryoscopic constant.Solution in Tamil
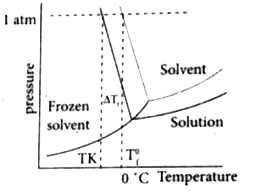
(i) Freezing point is difined as the temperature at which the solid and the liquid states of the substances have the same vapour pressure.
(ii) When a non volatile solute is added to water at its freezing point, the freezing point of water is lowered from 0∘C. The lowering of freezing point of the solvent when a soute is added is called depression in freezing point ΔTf.
(iii) ΔTf=T∘f−Tf
Topper's Solved these Questions
Similar Questions
(i) Define solution.
(ii) Explain the types of solutions with suitable example.
View SolutionCalculate the mole fraction of benzene is solution containing 30% by mass in carbon tetrachloride.
View SolutionWrite short note an freezing point depression in freezing point and cryoscopic constant.
View SolutionDepression in freezing point or cryoscopy
View SolutionWhat is the other name for freezing point depression constant or molal depression constant ?
View Solutionहिमांक बिन्दु की कमी जो निम्न में से कौन-से विलयन जल के क्रायोस्कोपिक स्थिरांक को प्रदर्शित करता है?
View SolutionDepression of freezing point of which of the following solutions does represent the cryoscopic constant of water ?
View SolutionExplain depression in freezing point. How depression in freezing point can be used to find molar mass of a non-volatile solute? Define molal depression constant.
View Solution

