For the following bond cleavages use curved-arrows to show the electron flow and classify each as homolytic or heterolytic fission. Identify reactive intermediate produced as free radical, carbocation and carbanion ?

Topper's Solved these Questions
- SAMPLE PAPER-8Book:FULL MARKSChapter:SAMPLE PAPER-8Exercise:Part-IV
Explore 10 Videos
- SAMPLE PAPER-8Book:FULL MARKSChapter:SAMPLE PAPER-8Exercise:Part-II
Explore 9 Videos
- SAMPLE PAPER 2 (SOLVED)Book:FULL MARKSChapter:SAMPLE PAPER 2 (SOLVED)Exercise:PART-IV
Explore 10 Videos
- SOLUTIONS Book:FULL MARKSChapter:SOLUTIONS Exercise:ADDITIONAL QUESTIONS SOLVED - NUMERICAL PROBLEMS
Explore 15 Videos
Similar Questions
(i) Balnce the following equations by ion electron method. KMnO4+SnCl2+HCl→MnCl2+SnCl4+H2O+KCl
(ii) Boric acid, H3BO3 is a mild antiseptic and often used as eye wash. A sample contains 0.543 mol H3BO3. What is the mass of boric acid in the sample?
View SolutionDefine Avogadro Number.
View Solution(i) The stabilization of a half filled d-orbital is more pronounced than that of the p-orbital why?
(ii) What are degenerate orbitals?
View SolutionCalculate the mole fraction of benzene is solution containing 30% by mass in carbon tetrachloride.
View SolutionFor the following bond cleavages, use curved-arrow to show the electron flow and classify each as homolysis or heterolysis. Identify intermediate products as free radical, carbocation, and carbanion. (a) CH_3O - OCH_3 rarr CH_3 dot O+ dot O CH_3 .
View SolutionClassify each of the following as homolysis as homolysis or heterolysis. Identify the reaction intermediates produced , as free radical, carbocation and carbanion.
View SolutionFor the following bond cleavages, use curved arrows to show the electron flow and classify each as homolysis or heterolysis. Identify reactive intermediate produced during the fission.
View Solutionनिम्नलिखित आबन्ध विदलनों के लिए इलेक्ट्रॉन - विस्थापन को मुड़े तीरों दर्शाइए तथा प्रत्येक विदलन को समांश अथवा विशमांश में वर्गीकृत कीजिए। साथ ही निर्मित सक्रिय मध्यवर्ती उत्पादों में मुक्त-मूलक, कार्बधनायन तथा कार्बऋणायन पहचानिए :
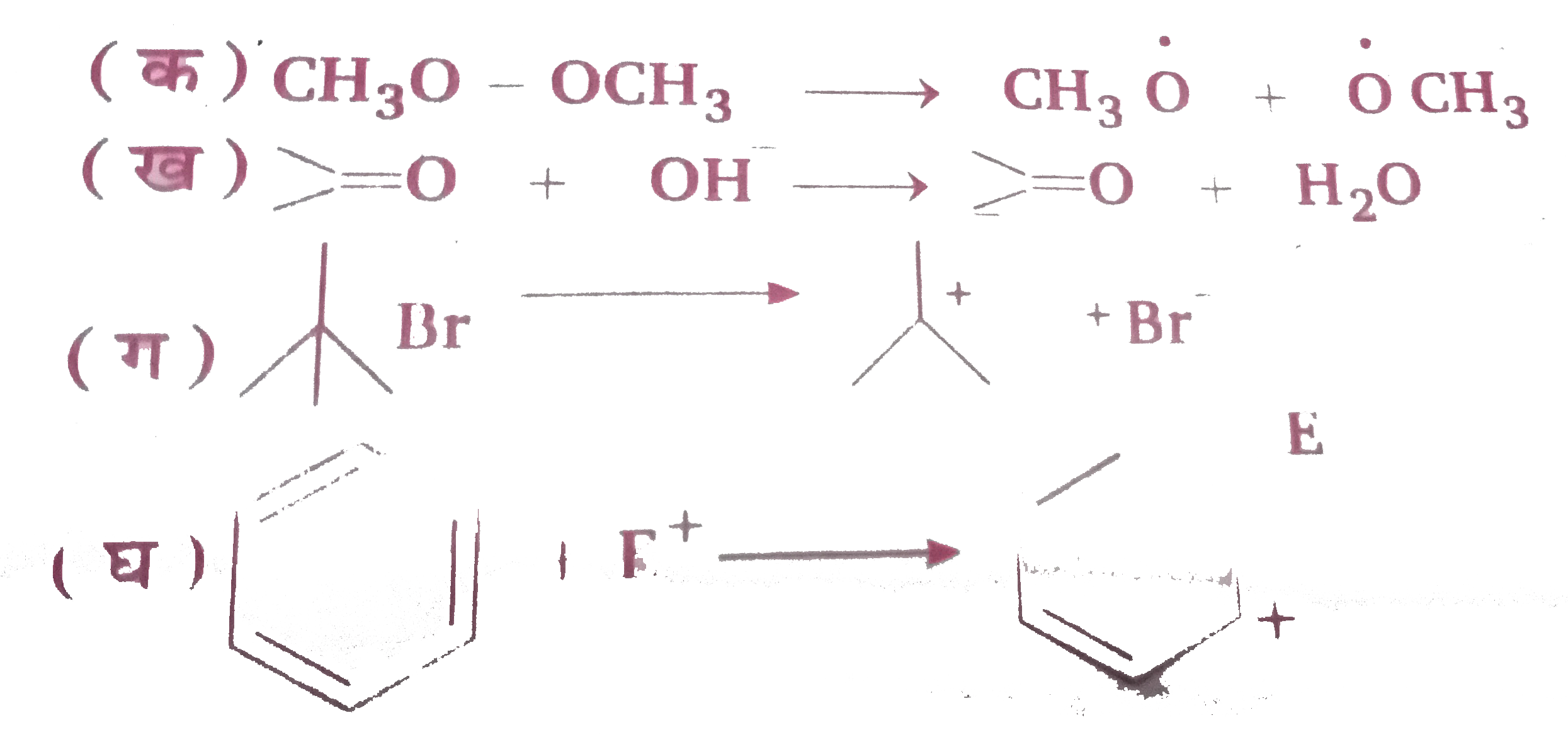
View SolutionFor the given bond cleavages, use curved - arrows to show the electron flow and classify each as homolysis or heterolysis. Identify reactive intermediate produced as free radical, carbocation and carbanion. CH_(3)O-OCH_(3)rarrCH_(3)overset(*)(O)+overset(*)(O)CH_(3)
View SolutionCorrect statement is :- (1) Homolytic bond fission generates free radicals (2) Carbocation has incomplete octet. (3) If shared electron pair is unequally distributed then it is called heterolytic bond fission. (4) All of these
View SolutionFor the following bond cleavages, use curved-arrows to show the electron flow and classify each as homolysis or heterolysis. Identify reactive intermediate produced as free radical, carbocation and carbanion. (a) CH_(3)O-OCH_(3)rarrCH_(3)overset(.)O+overset(.)(O)CH_(3) (b)
View SolutionHomolytic fission of a covalent bond leads to the formation of
(a) electrophile
(b) nucleophile
(c) free radical
(d) carbocation
(e) carbanion
View SolutionFor the following bond cleavages, use curved-arrow to show the electron flow and classify each as homolysis or heterolysis. Identify intermediate products as free radical, carbocation, and carbanion.
(a) CH3O−OCH3→CH3.O+.OCH3
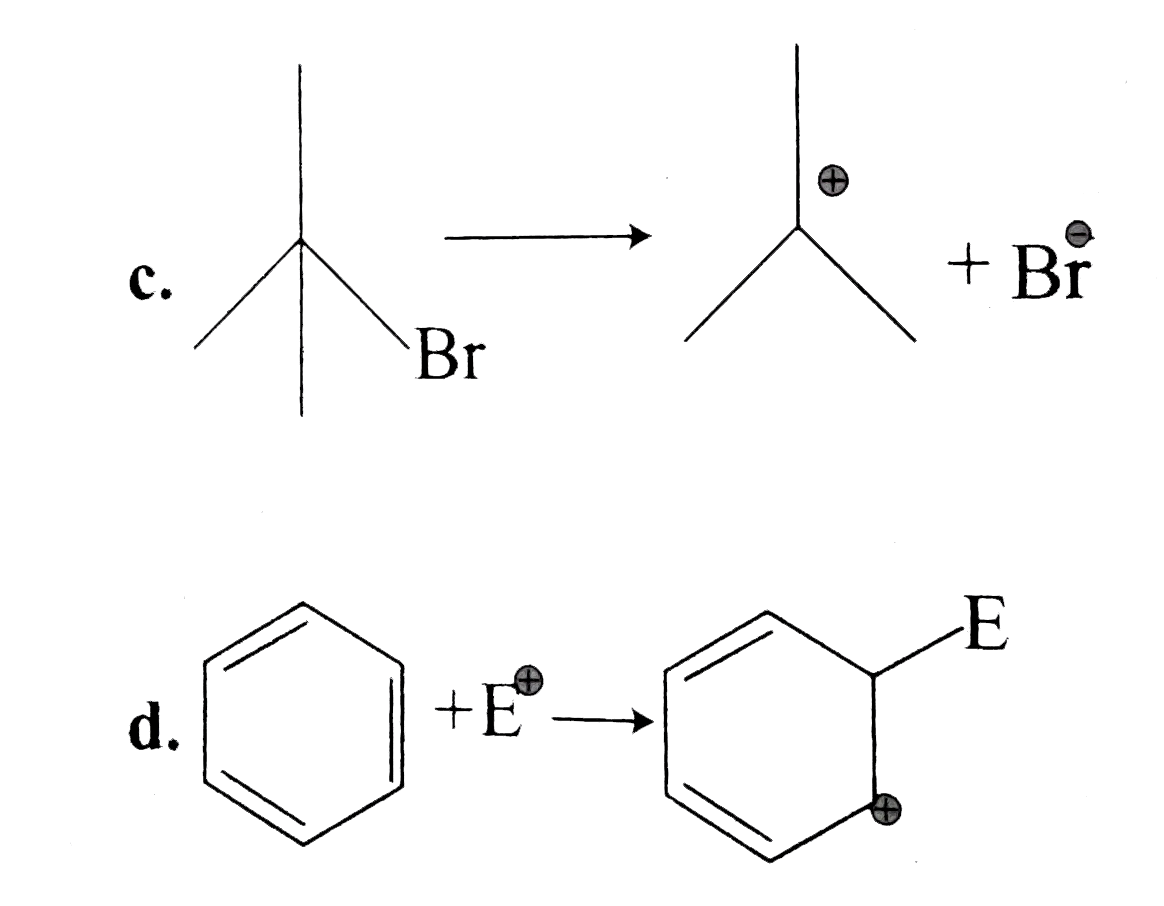 .
.
View SolutionFor the following bond cleavages, use curved arrows to show the electron flow and classify each as homolysis or heterolysis. Identify reactive intermediate produced as free radical, carbocation and carbanion (a) CH_(3)O- OCH_(3) rarr CH_(3) overset(.)(O) + overset(.)(O)CH_(3)
View SolutionFor the following bond cleavages, use curved arrows to show the electron flow and classify each as homolysis or heterolysis. Identify reactive intermediate produced as free radical, carbocation and carbanion
(a) CH3O−OCH3→CH3.O+.OCH3
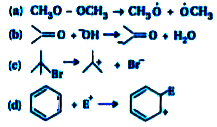
View Solution(i) Only carbocation form by homolytic cleavage of bond. (ii) By heterolytic cleavage of bond, the carbocation or carbanion are formed. (iii) The carbon of carbanion is sp^(2) and carbon of carbocation is sp^(3) . (iv) Carbon of carbanion is sp^(3) and carbon of carbocation is sp^(2)
View Solution


