Explin about inductive effect.
(ii) What do you mean by conformation ? Explain about staggered conformation in ethane .
Text Solution
It is defined as the change in the polarization of a covalent bond due to the presence of adjacen bonded atoms or groups in the molecule. It is denoted as I-effect .
2 . Atoms or groups which lose electron towards a carbon atom are said to have a + I effect.
Example CH3−,(CH3)2CH−,(CH3)2C etc .
3 Atoms or groups which draw electrons away from a carbon atom are said to have a- I effect.
Example , −NO2,−I,−Br,−OH,C6H5 etc.
For example, consider ethane and ethyl chloride. The C-C bond in ethane is non- polar while the C-C bond in ethyl chloride is polar. We know that chlorine is more electronegative than carbon and hence it attracts the shared pair of electrons between C-Cl in ethyl chloride towards itself.
δδ+C2H3→−δC1H2→−δCl
This develops a slight negative charge on chlorine and a slight positive charge on carbon to which chlorine is attached. To compensate it, the C1 draws the shared pair of electron between itself and C2. This polarization effect is called inductive effect.
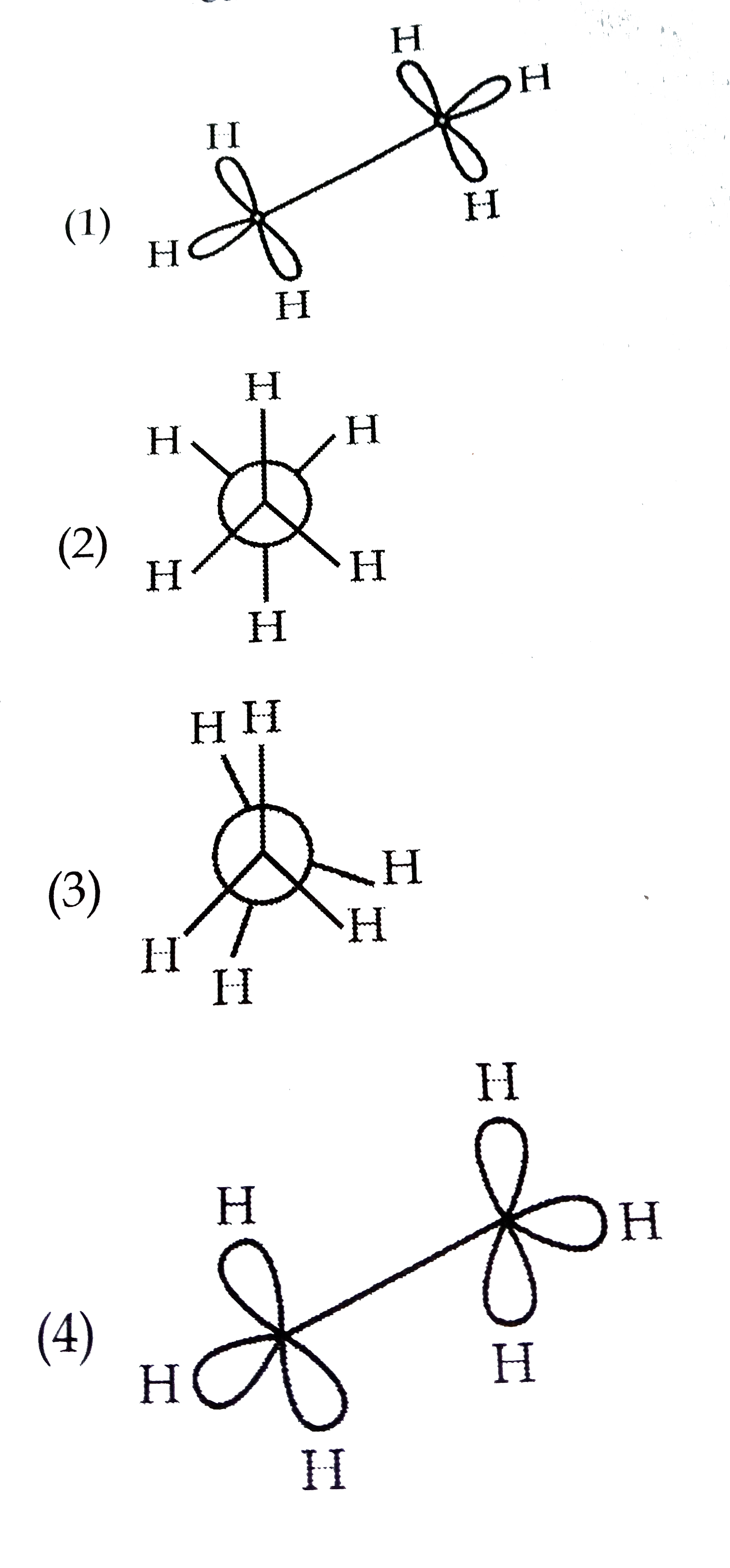
(ii) The rotation about C-Csingle bondaxis yielding several arrangements of a hydrocarbon called conformers.
Staggered conformation: In this conformation, the hydrogens of both the carbon atoms are far apart from each other. The repulsion between the atoms is minimum and it is the most stable conformer.

|
Topper's Solved these Questions
- EXAMINATION QUESTION PAPER - JUNE 2019Book:FULL MARKSChapter:EXAMINATION QUESTION PAPER - JUNE 2019Exercise:PART-III
Explore 11 Videos
- ENVIRONMENTAL CHEMISTRY Book:FULL MARKSChapter:ENVIRONMENTAL CHEMISTRY Exercise:ADDITIONAL QUESTIONS SOLVED ( 5-Mark Questions )
Explore 8 Videos
- EXAMINATION QUESTION PAPER - MARCH 2019Book:FULL MARKSChapter:EXAMINATION QUESTION PAPER - MARCH 2019Exercise:PART-IV
Explore 10 Videos
Similar Questions
Knowledge Check
The eclipsed and staggered conformation of ethane is due to -
AFree rotation about C-C single bondBRestricted rotation about C - C single bondCAbsence of rotation about C - C bondDNone of the aboveThe eclipsed and staggered conformation of ethane is due to -
AFree rotation about C-C single bondBRestricted rotation about C-C single bondCAbsence of rotation about C-C bondDNone of the aboveThe correct statement the comparison of staggered and eclipsed conformations of ethan is:
AThe staggered conformation of ethane is less stable than eclipsed conformation, because staggered conformation has torsional strainBThe eclipsed conformation of ethane is more stable than staggered conformation, because eclipsed conformation has not torsional strainCThe eclipsed conformation of ethane is more stable than staggered conformation even through the eclipsed conformation has torsional strainDThe staggered conformation of ethane is more stable than eclipsed conformation, because staggered conformation has no tosional strain
What do you know about Augustus? Explain.
View SolutionWhat do you mean about Summer Solstice?
View SolutionThe dihedral angle between the hydrogen atoms of two methyl groups in staggered conformation of ethane is
View SolutionIn the following structures which two forms are staggered conformation of ethane?
View SolutionWhich of the following are correct chain isomers of butane ?
(i)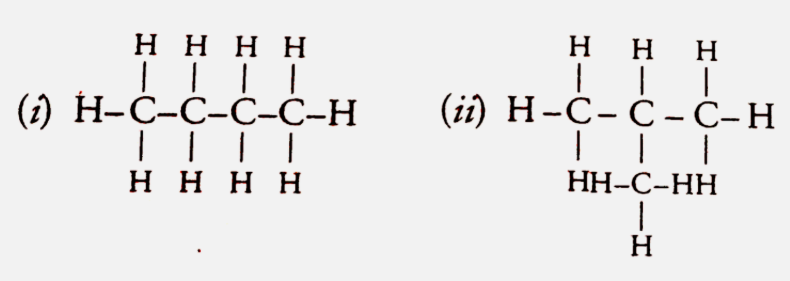
View Solution

