Draw all possible geometrical isomers of the complex [Co(en)2Cl2]+ and identify the optically active isomer.
Text Solution
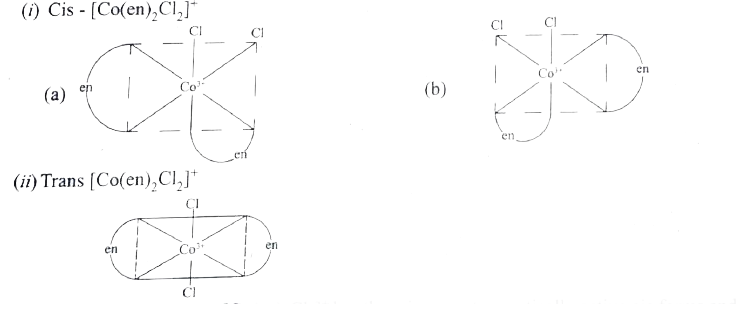
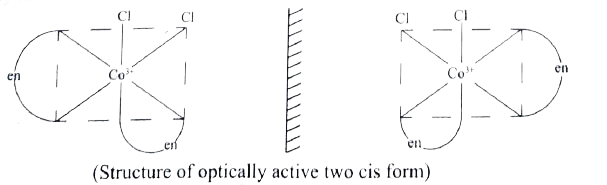
The coordination complex [Co(en)2Cl2]+ has three isomers two optically active cis forms and the optically inactive trans form .
|
Similar Questions
Knowledge Check
The possible geometric isomers of the complex , [Co(en)Br2Cl2]− is :
A2B3C4D6
Draw the geometrical isomers of complex [Pt(en)2Cl2]2+ .
View SolutionDraw one of the geometrical isomers of the complex [Pt(en)2Cl2]2+ which is optically active.
View SolutionDraw one of the geometrical isomers of the complex [Pt(en)2Cl2]2+ which is optically inactive.
View SolutionDraw the geometrical isomers of complex [Pt(NH3)2Cl2].
View SolutionDraw the geometrical isomers of but-2-ene.
View SolutionDraw one of the geometrical isomers of the complex [Pt(en)2Cl2]2+ which is optically active.
View SolutionDraw one of the geometrical isomers of the complex [Pt(en)2Cl2]2+ which is optically inactive . Also write the name of this entity according to the IUPAC nomenclature .
View Solution

