Square planar complexes have………… type of hybridisation.
sp3
dsp2
sp3d
sp3d2
The correct Answer is:B, D
Topper's Solved these Questions
- COORDINATION CHEMISTRYBOOK - FULL MARKSCHAPTER - COORDINATION CHEMISTRYEXERCISE - Additional Question(Fill in the blanks)
64 Videos
- COORDINATION CHEMISTRYBOOK - FULL MARKSCHAPTER - COORDINATION CHEMISTRYEXERCISE - Additional Question(Match the following)
10 Videos
- COORDINATION CHEMISTRYBOOK - FULL MARKSCHAPTER - COORDINATION CHEMISTRYEXERCISE - Textbook Evaluation
50 Videos
- CHEMISTRY IN EVERYDAY LIFE BOOK - FULL MARKSCHAPTER - CHEMISTRY IN EVERYDAY LIFE EXERCISE - ADDITIONAL QUESTION ( 5 Mark Questions )
2 Videos
- ELECTRO CHEMISTRYBOOK - FULL MARKSCHAPTER - ELECTRO CHEMISTRYEXERCISE - ADDITIONAL QUESTIONS -5- MARK QUESTIONS
15 Videos
Similar Questions
The reaction between Ferric chloride and potassium thio cyanate solution gives a blood red coloured coordination compound as ……………
View Solution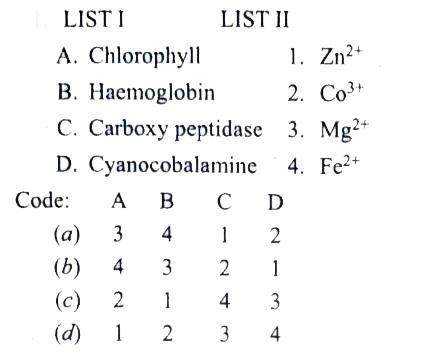
View SolutionThe sum of primary valance and secondary valance of the metal M in the complex [M(en)2(Ox)]Cl is …………….. .
View SolutionExplain free radical polymerisation with example.
View SolutionHow would you measure the conductivity of ionic solutions?
View SolutionA square planar complex is formed by hybridisation of which atomic oritals?
View SolutionA square planar complex is formed by hybridisation of which atomic orbitals?
View SolutionWhat type of hybridisation is involved in the geometry of the i) square planar ii) tetrahedral iii) octahedral complexes?
View SolutionIf the geometry of the complex [PtCl_(4)]^(2-) is square planar, which type of orbitals are involved in its hybridisation?
View SolutionA square planar complex is formed by hybridisation of which atomic orbitals?
View SolutionA square planar complex is formed by hybridisation of which atomic orbitals ?
View SolutionWhich of the following dsp^(2) hybridised complex ions is square planar-
View SolutionThe complex having square planar geometry is
View SolutionA: All square planar complexes can exhibit geometrical isomerism.
R: In square planar complexes metal assumes sp3 hybridisation.
View SolutionA square planar complex is formed by hybridisation of which of the following atomic orbitals ?
View Solution

