The d orbital involved in dSP3 hybridisation of [Fe(CO)5] is………..
dxy
dyz
dxz
dx2−y2
The correct Answer is:B, D
Topper's Solved these Questions
- COORDINATION CHEMISTRYBOOK - FULL MARKSCHAPTER - COORDINATION CHEMISTRYEXERCISE - Additional Question(Fill in the blanks)
64 Videos
- COORDINATION CHEMISTRYBOOK - FULL MARKSCHAPTER - COORDINATION CHEMISTRYEXERCISE - Additional Question(Match the following)
10 Videos
- COORDINATION CHEMISTRYBOOK - FULL MARKSCHAPTER - COORDINATION CHEMISTRYEXERCISE - Textbook Evaluation
50 Videos
- CHEMISTRY IN EVERYDAY LIFE BOOK - FULL MARKSCHAPTER - CHEMISTRY IN EVERYDAY LIFE EXERCISE - ADDITIONAL QUESTION ( 5 Mark Questions )
2 Videos
- ELECTRO CHEMISTRYBOOK - FULL MARKSCHAPTER - ELECTRO CHEMISTRYEXERCISE - ADDITIONAL QUESTIONS -5- MARK QUESTIONS
15 Videos
Similar Questions
The reaction between Ferric chloride and potassium thio cyanate solution gives a blood red coloured coordination compound as ……………
View Solution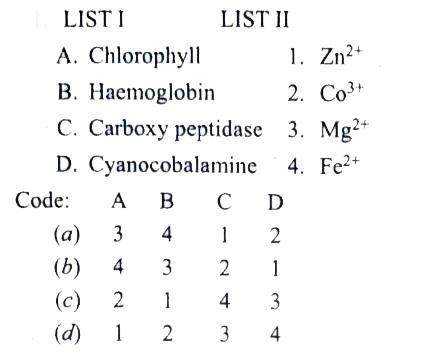
View SolutionThe sum of primary valance and secondary valance of the metal M in the complex [M(en)2(Ox)]Cl is …………….. .
View SolutionExplain free radical polymerisation with example.
View SolutionHow would you measure the conductivity of ionic solutions?
View SolutionAs PF5 molecule is sp3 d hybridised and is trigonal bipyramidal (TbP) shape Which d-orbital is involved in sp3 d hyridisation .
View SolutionThe d-orbital involved in sp^3d hybridisation is
View SolutionThe d-orbitals involved in dsp^2 hybridisation is
View SolutionThe orbitals of iron involved in the hybridization in Fe(CO)5 are
View SolutionWhich d-orbital is involved in dsp^(2) hybridisation why ?
View SolutionFor the complexes showing the square pyramidal structure, the d-orbital involved in the hybridisation is:
View SolutionFor the complexes showing the square pyramidal structure, the d-orbital involved in the hybridisation is:
View SolutionThe d orbital involved in the dsp^(3) hybridisation of Fe(CO)_(5)] is ...............
View SolutionIn the octahedral complexes, if the (n−1)d orbitals are involved in hybridisation, they are called..............and..........complexes.
View SolutionThe d - orbitals involved in sp3d2 hybridisation are ……………..and …………….
View Solution

