Explain about the geometrical isomerism in complexes having coordination number 4.
(i) Geometrical isomerism exists in heteroleptic complexes due to different possible three diamensional spatial arrangements of the ligands around the central metal atom . This type of isomerism exist in square planar tetrahedral complexes.
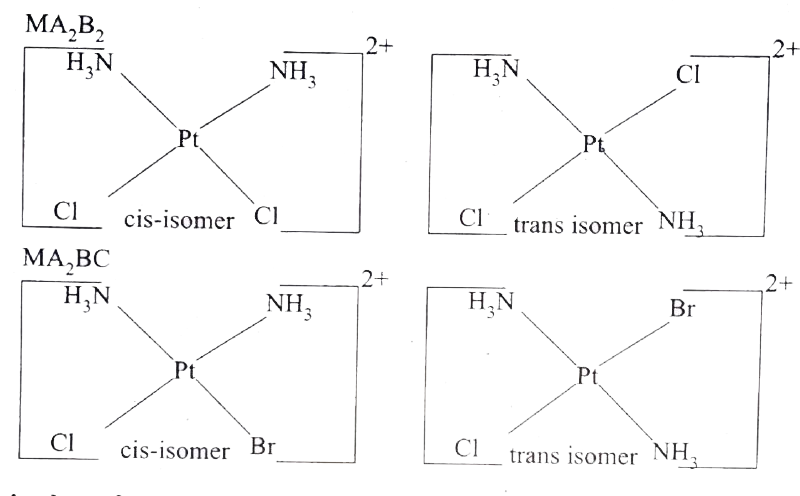
(ii) In square planar complexes of the form [MA2B2]n± and [MA2BC]N+ where A,B and C are monodentate ligands and M is the central metal ion /atom.
(iii) Similar groups (A or B) present either on same side or on the opposite side of the central metal atom (M) give rise to two different geometrical isomers and they are called cis and trans isomers respectively.
(iv) The square planar complex of the [M(XY)2]n± where XY is a bidentate ligand with two different coordinating atom also shows cis-trans isomerism .
(v) Square planar complex of the form [MABCD]n+ also shows cis-trans isomerism. In this case, by considering any one of the ligands [A,B,C,D] as a reference , the rest of the ligands can be arranged in three different ways leading to three geometrical isomers.
Similar Questions
Assertion (A) Complexes of MX6 and MX5L type (X and L are unidentate) do not show geometrical isomerism.
Reason (R) Geometrical isomerism is not shown by complexes of coordination number 6.
View SolutionAssertion (A) Complexes of MX6 and MX5L type (X and L are unidentate) do not show geometrical isomerism.
Reason (R) Geometrical isomerism is not shown by complexes of coordination number 6.
View Solution
Knowledge Check
Assertion (A) Complexes of MX6 and MX5L type (X and L are unidentate) do not show geometrical isomerism.
Reason (R) Geometrical isomerism is not shown by complexes of coordination number 6.AAssertion and reason both are true, reason is correct explanation of assertion.BAssertion and reason both are true but reason is not the correct explanation of assertion.CAssertion is true, reason is false.DAssertion is false, reason is true.Statement-1: Complexes of MX6 and MX5L type (X and L are unidentate) do not show geometrical isomerism.
Statement-2: Geometrical isomerism is not shown by complexes of coordination number 6.AStatement-1 is True, Statement-2 is True , Statement-2 is a correct explanation for Statement-1.BStatement-1 is True, Statement-2 is True, Statement-2 is NOT a correct explanation for Statement-1.CStatement-1 is True, Statement-2 is False.DStatement-1 is False, Statement-2 is True.Statement-1: Complexes of MX6 and MX5L type (X and L are unidentate) do not show geometrical isomerism.
Statement-2: Geometrical isomerism is not shown by complexes of coordination number 6.AStatement-1 is True, Statement-2 is True , Statement-2 is a correct explanation for Statement-1.BStatement-1 is True, Statement-2 is True, Statement-2 is NOT a correct explanation for Statement-1.CStatement-1 is True, Statement-2 is False.DStatement-1 is False, Statement-2 is True.
Geometrical and Optical Isomerism in Complex having CN 6
View SolutionGeometrical isomerism is found in coordination compounds having coordination number:
View SolutionAssertion: Geometrical isomerism is shown by complexes with coordination number 6.
Reason: Because it has d2sp3 type hybridisation.
View SolutionGeometrical isomerism is found in coordination compounds having coordination number :
View SolutionGeometrical isomerism is found in coordination compounds having coordination number:
View Solution

