In which of the following, first memeber is more stable than second?
A
(C6H5)2∗CCH3,(C6H5)3∗C
B
:CH2,⋅CH2⋅
C
(C6H5)3+C,+CH3
D
(C2H5)3,∗C,(CH3)3∗C
Video Solution
Text Solution
The correct Answer is:C
(C6H5)3−C is more stable than −CH3 because three phenyl groups not only decrease the electron density but also stabilise it by the phenomena of resonance.
|
Similar Questions
Knowledge Check
In which of the following pairs, first species is more stable than second ?
ACH3CH2O− or CH3O∣∣CO−BCH3O∣∣C−CHCH2O∣∣CH or CH3O∣∣C−CHO∣∣CH3CCH3−CHCH2O∣∣CCH3 or CH3CH2−CHO∣∣CCH3D
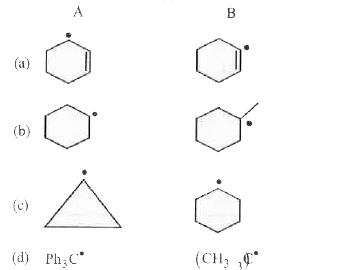
In which of the following pairs A is more stable than B ?
A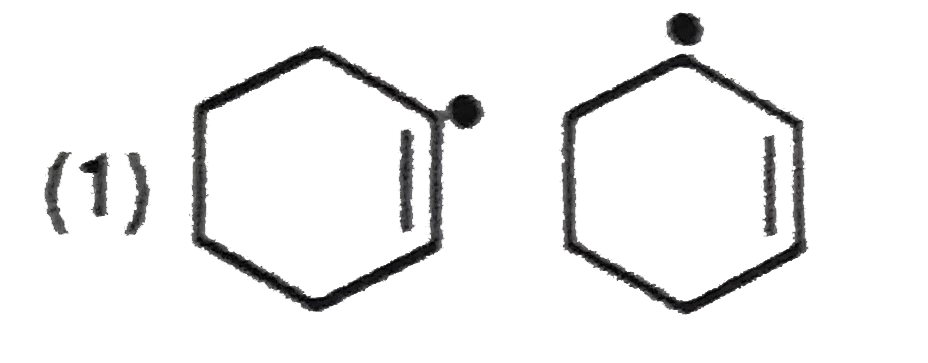 B
B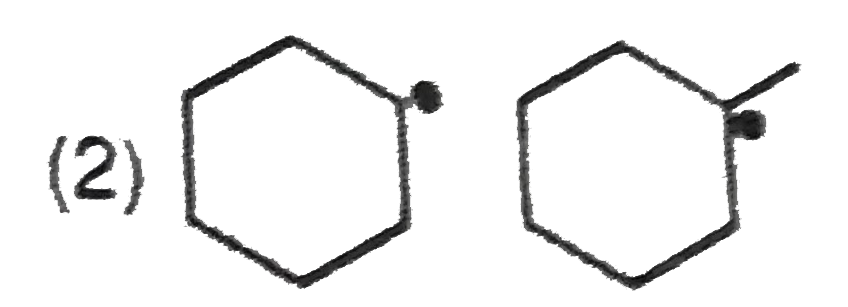 C
C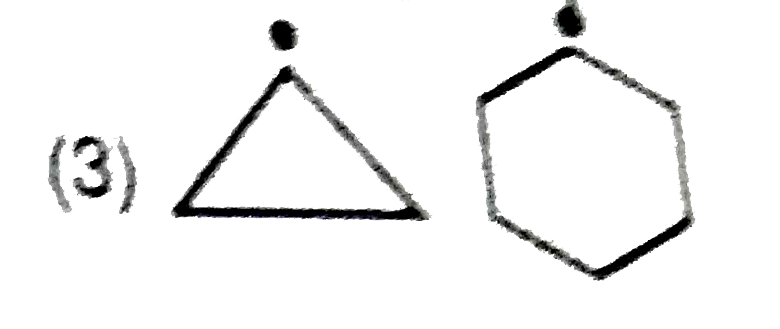 DPh3C⋅,(CH3)C⋅
DPh3C⋅,(CH3)C⋅In which of the following pairs A is more stable than B?
A B
B C
C D
D
In which of the following pairs A is more stable than B?
View SolutionIn which of the following pairs II is more stable than I
View SolutionIn which of the following 2nd anion is more stable than first?
View SolutionIn which of the following first is more basic than second
View SolutionIn which of the following first is more acidic than second ?
View Solution