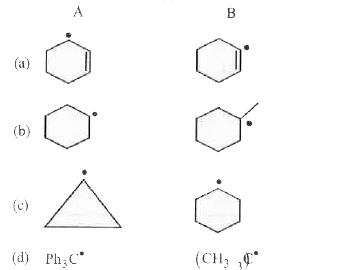
In which of the following 2nd anion is more stable than first?
A
O_(2)N-overset(Ɵ)CH_(2) and F-overset(Ɵ)CH_(2)
B
ƟCF3andƟCCl3
C
F3C−ƟCH2andCl3C−ƟCH2
D
CH3−O∣∣C−ƟCH2andH2N−ƟCH2
Video Solution
Text Solution
The correct Answer is:B
ƟCCl3 is more stabilise than (Ɵ)CF3 because of d-orbital resonance.
|
Similar Questions
Knowledge Check
In which of the following 2nd anion is more stable than first ?
AO2N−ɵCH2 and F−CH2BɵCF3 and ɵCCl3CF3C−ɵCH2 and Cl3C−ɵCH2DCH3−O∣∣C−ɵCH2 and H2N−ɵCH2In which of the following pairs A is more stable than B?
A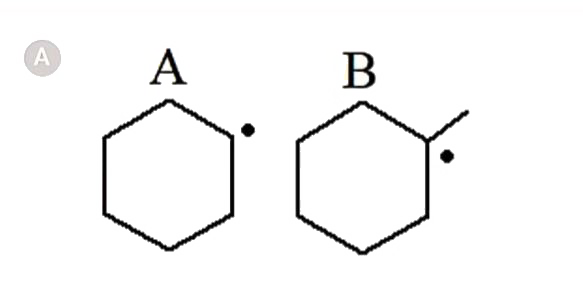 B
B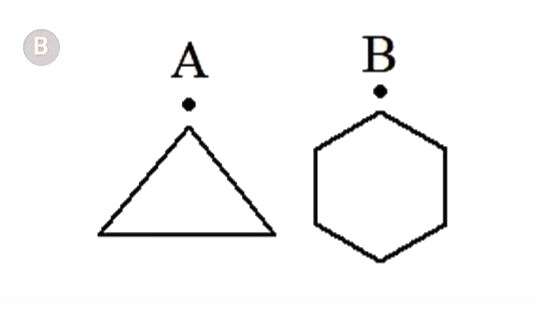 C
C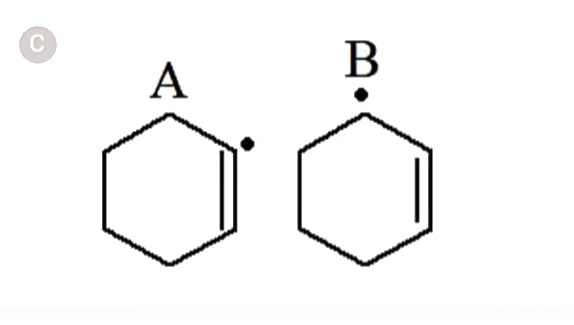 DABPh3C.(CH3)3C.
DABPh3C.(CH3)3C.In which of the following pairs II is more stable than I
A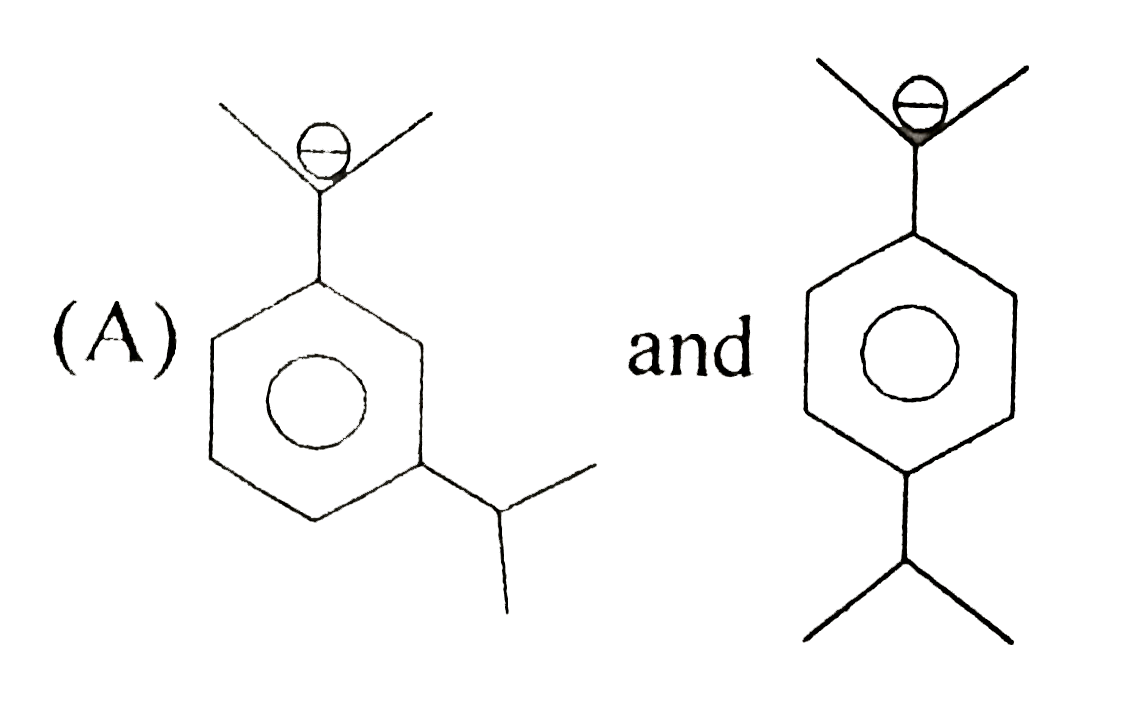 BCH3−⊕C=OandCH3−C≡⊕OC
BCH3−⊕C=OandCH3−C≡⊕OC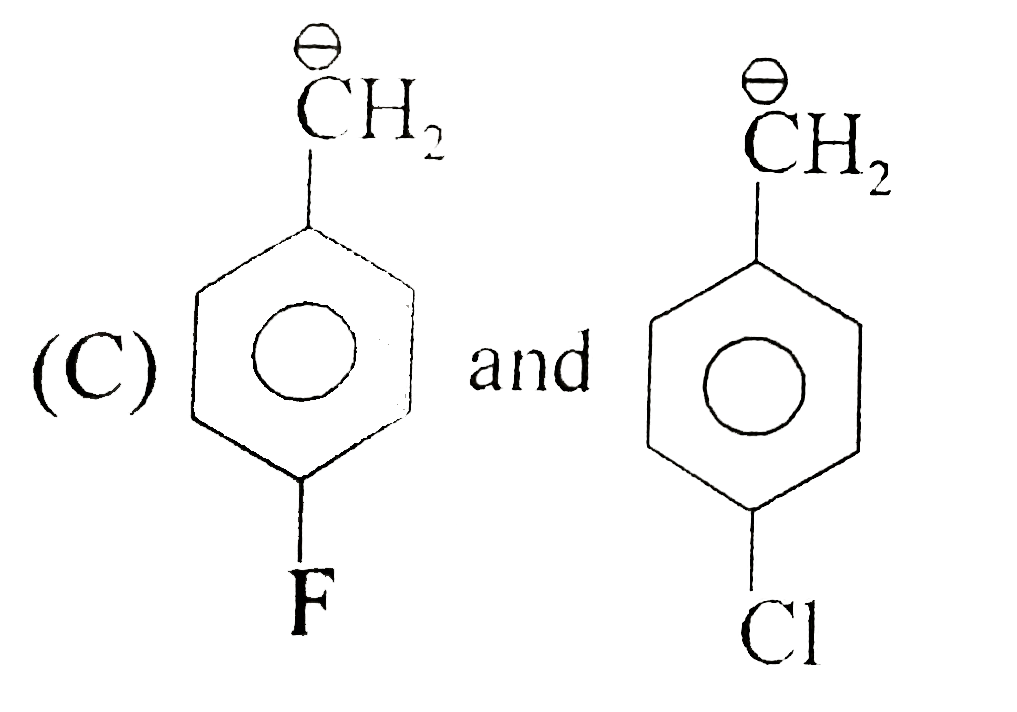 DCH3−⊕CH2 & CD3−⊕CH2
DCH3−⊕CH2 & CD3−⊕CH2
Which of the following ions is more stable ?
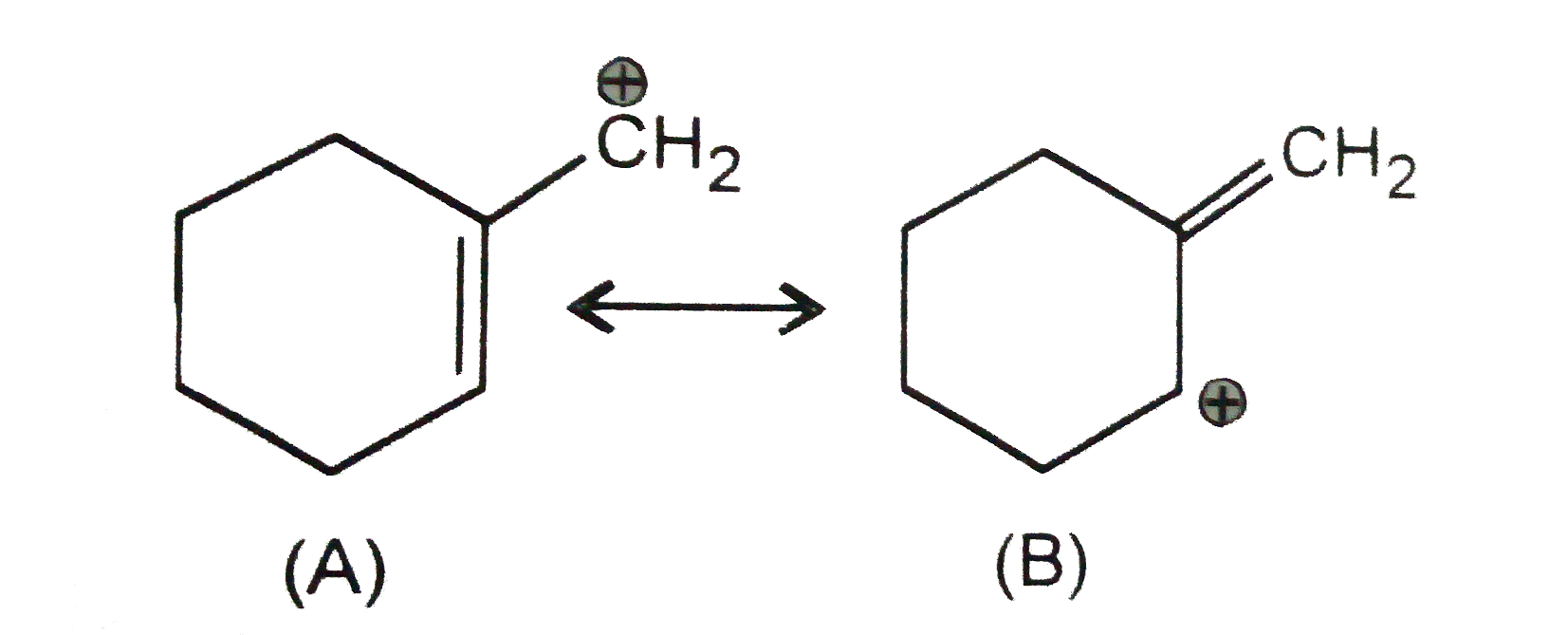
View SolutionWhich of the following ions is more stable ?
View SolutionIn which of the following pairs A is more stable than B?
View SolutionIn which of the following pairs A is more stable than B ?
View SolutionWhich of the following is second ion is more stable than the first
View Solution