By which reaction best yield of H2 gas forms:
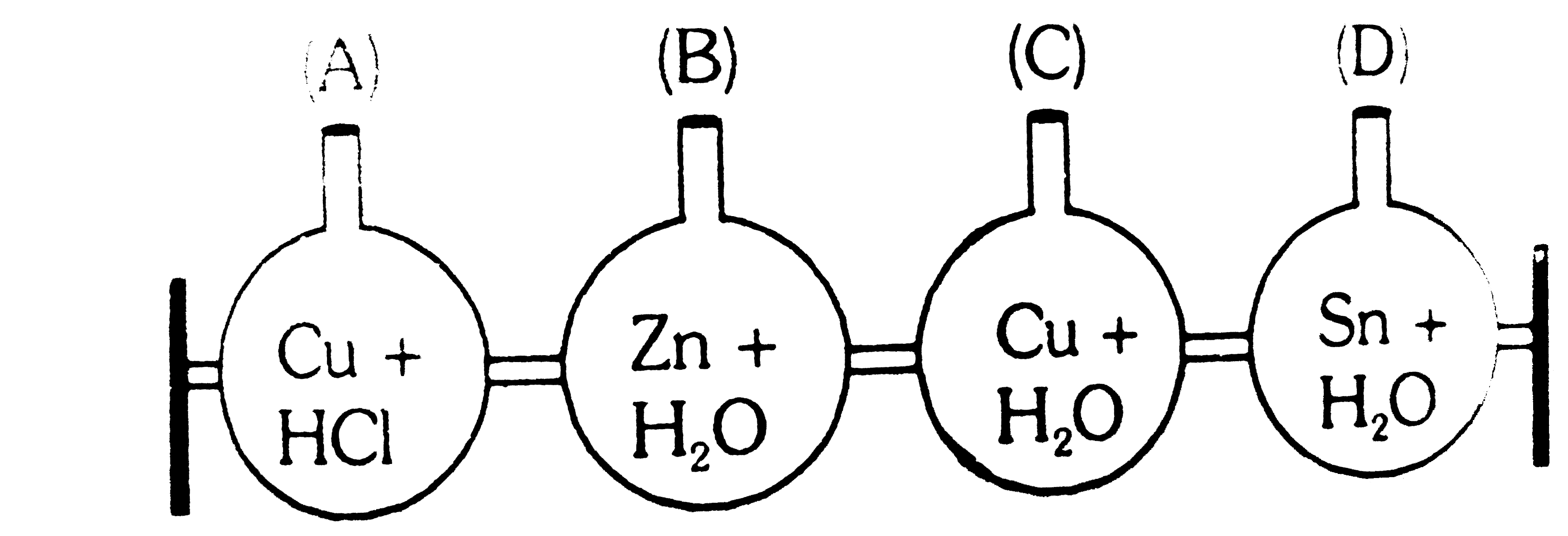
temperature of all flask More then 100∘C
Only By D
Only By A & B
Only By A, B & C
In all process
The correct Answer is:A
Sn react with water to produce hydrogen gas.
Similar Questions
The Kc for A2(g)+B2(g)⇔2AB(g) at 100∘C is 50. If one litre flasks containing one mole of A2 is connected with a two litre flask containing 2mole of B2, how many mole of AB will be formed at 100∘C?
View SolutionAn evacuated bulb of unknown volume is filled with a sample of H2 gas at a temperature T The pressure of the gas in the bulb is 756mm Hg A portion of the H2 gas is transferred to a different flask and found to occupy a volume of 40.0mL at 1.00 atn and the same temperature T The pressure of the H2 gas remaining in the original bulb drops to 625mm hg at the same temperature T Assuming H2 is an ideal gas, what is the volume of the bulb ? .
View Solution
Knowledge Check
By which reaction. Best yield of H2 gas forms :
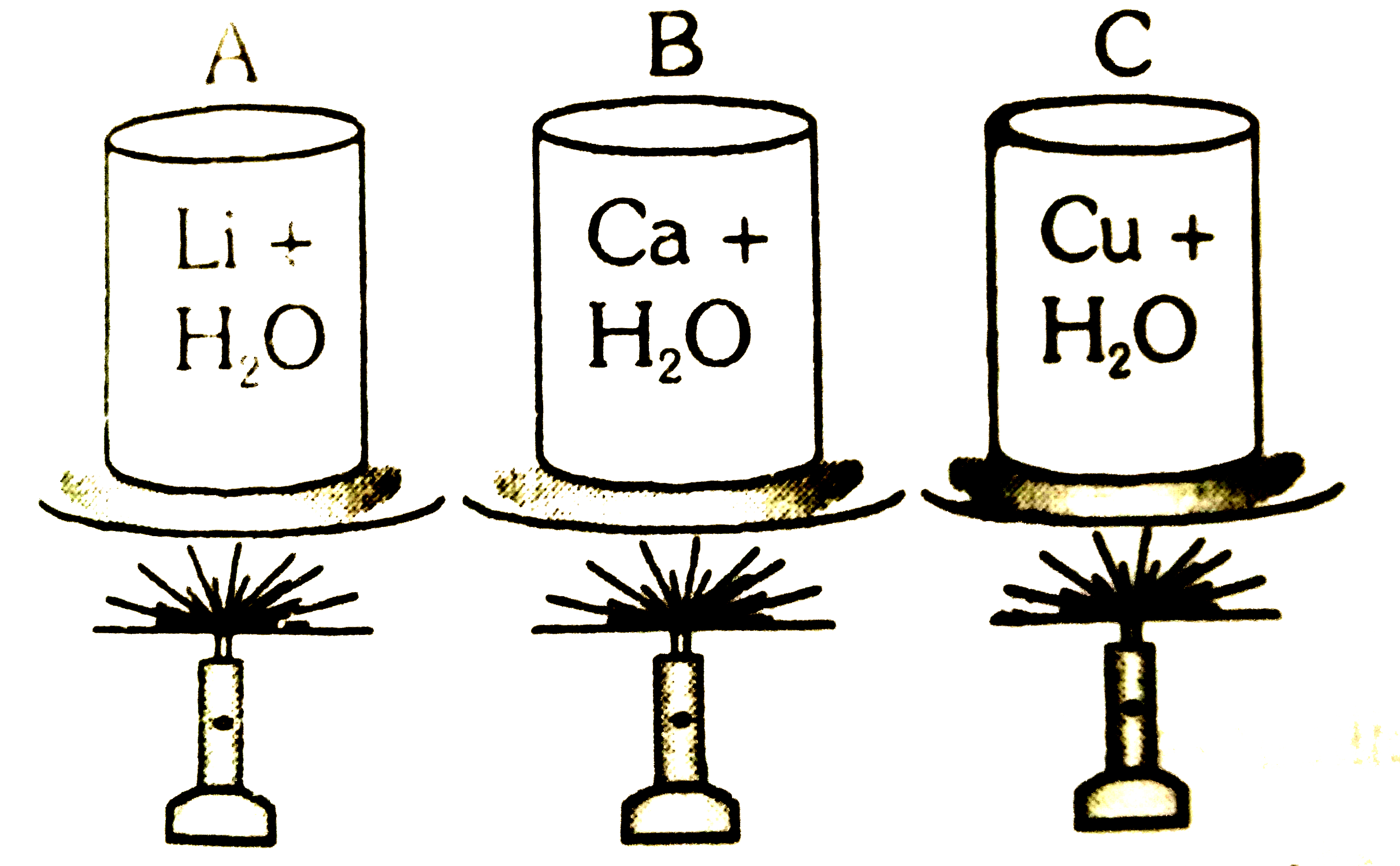
Temperature of container is more than 100∘CABy C onlyBBy A, B onlyCBy A, B, C onlyDNone of theseWhich process does not form H2 gas.
AZn+ice cold H2OBZn+H2SO4CNa+ice cold H2ODMh+HotH2OWhich of the following reaction gives H2 gas ?
ANaNH2+H2O→BZn+conc.H2SO4→CZn+ aqNaOH→DCaH2+H2O→
Which of following reaction do not form H2?
View SolutionThe reaction conditions leading to best yields of C2H5Cl are :
View SolutionThe reaction condtions leading to best yields of C2H5CI are
View SolutionA flask is filled with 13g of an ideal gas at 27∘C and its temperature is raised to 52∘C. The mass of the gas that has to be released to maintain the temperature of the gas in the flask at 52∘C, the pressure remaining the same is
View SolutionIf 4 litre of H2 gas at 400 mm Hg and 47∘C is transferred to 19 litre flask at 107∘C. Then pressure of H2 gas is:
View Solution

