By which reaction. Best yield of H2 gas forms :
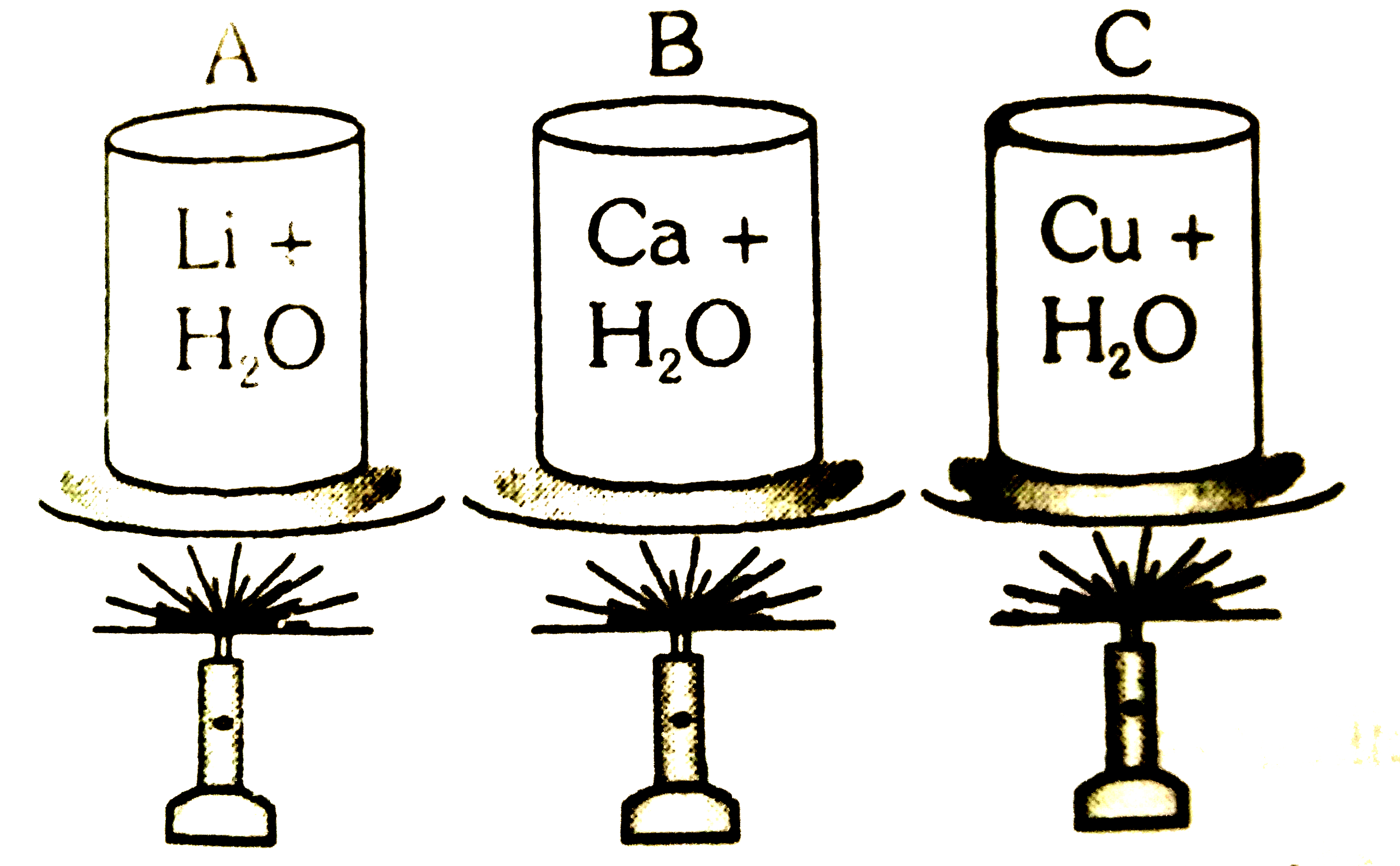
Temperature of container is more than 100∘C
By C only
By A, B only
By A, B, C only
None of these
The correct Answer is:D
Similar Questions
Knowledge Check
By which reaction best yield of H2 gas forms:
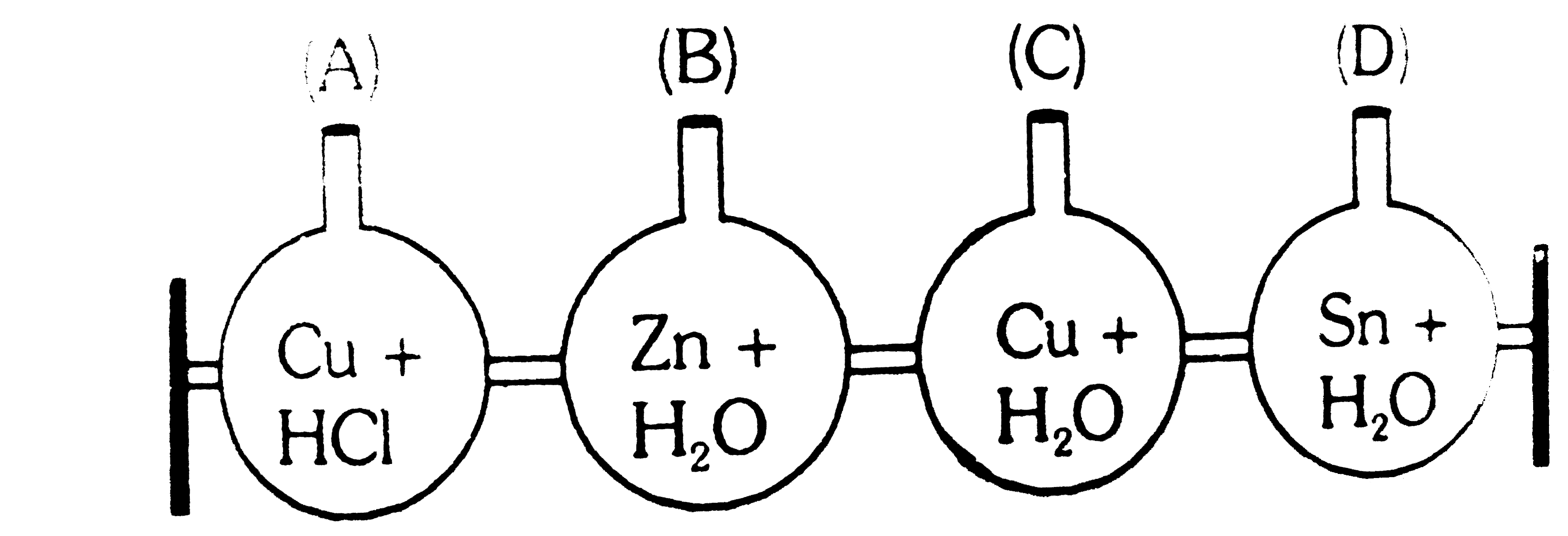
temperature of all flask More then 100∘CAOnly By DBOnly By A & BCOnly By A, B & CDIn all processThe reaction conditions leading to best yields of C2H5Cl are :
AC2H6(excess)+Cl2UV light −−−−−→BC2H6+Cl2Darkroom temp.CC2H6+Cl2(excess)UV light −−−−−→DC2H6+Cl2UV light −−−−−→The reaction condtions leading to best yields of C2H5CI are
AC6H6(excess)+CI2UV light−−−−−→BC6H6+CI2Dark−−−−−−→Room temp.CC2H6+CI2(excess)UV light−−−−→DC2H6+CI2UV light−−−−→
Is it possible to raise the temperature of water by heating it in a container made up of paper ? [Temperature required to burn the paper is more than 100∘C]
View SolutionAn element X of group 2 reacts with H2 gas at 200∘C to form compound Y. when Y is heated to a higher temperature, it decomposes to element X and H2 gas in the ratio of 559 ml of H2 (measured at STP) for 1.00 g of X reacted. X also combines with Cl2 to form a compound Z which contains 63.89 peerrcent mass of chlorine. identify X, Y and Z.
View SolutionAn element X reacts with H2 gas at 200∘C to form compound Y. when Y is heated to a higher temperature, it decomposes to element X and H2 gas in the ratio of 559 ml of H2 (measured at STP) for 1.00 g of X reacted. X also combines with Cl2 to form a compound Z which contains 63.89 peerrcent mass of chlorine. identify X, Y and Z.
View SolutionIn a reaction vessel, 100gH2 and 100 g Cl2 are mixed and suitable conditions are provided for the reaction: H2(g)+Cl2(g)→2HCl(g)
The amount of HCl formed (at 90% yield) will be
View SolutionMixing of N2 and H2 form an ideal gas mixture at roo, temperature in a container.For this process, which of the following statements is true?
View Solution

