4 moles of ..................
The correct Answer is:03.5
TVγ−1=C
γ−1=n
n=613
γ=4×72R+2×52R4×52R+2×32R=1913
Topper's Solved these Questions
- TEST PAPERBOOK - RESONANCECHAPTER - TEST PAPEREXERCISE - CHEMISTRY
37 Videos
- TEST PAPERBOOK - RESONANCECHAPTER - TEST PAPEREXERCISE - CHEMISTRY
37 Videos
- SEQUENCE & SERIES BOOK - RESONANCECHAPTER - SEQUENCE & SERIES EXERCISE - EXERCISE -2 (PART-II : PREVIOUSLY ASKED QUESTION OF RMO)
3 Videos
- TEST PAPERSBOOK - RESONANCECHAPTER - TEST PAPERSEXERCISE - MATHEMATICS
263 Videos
Similar Questions
Given that for a reaction of nth order, the integrated rate equation is:
K=1t(n−1)[1Cn−1−1Cn−10], where C and C0 are the concentration of reactant at time t and initially respectively. The t3/4 and t1/2 are related as t3/4 is time required for C to become C1/4) :
View SolutionGiven that for a reaction of nth order, the integrated rate equation is:
K=1t(n−1)[1Cn−1−1Cn−10], where C and C0 are the concentration of reactant at time t and initially respectively. The t3/4 and t1/2 are related as t3/4 is time required for C to become C1/4) :
View SolutionIn a book with page numbers from 1 to 100, sme pages are torn off. The sum of the numbers on the remaining pages is 4949. How many pages are torn off ?
View SolutionThe least positive integral value of 'k' for which there exists at least one line that the tangent to the graph of the curve y=x3−kx at one point and normal to the graph at another point is
View SolutionThe number of mole of oxalate ions oxidised by one mole of MnO_(4)^(-) ion is:
View Solution3 mole of FeSO_(4) are oxidised by a mole of KMnO_(4) in acidic medium whereas 3 moles of FeC_(2)O_(4) are oxidised by b mole of KMnO_(4) in acidic medium, the ratio of a and b is:
View Solution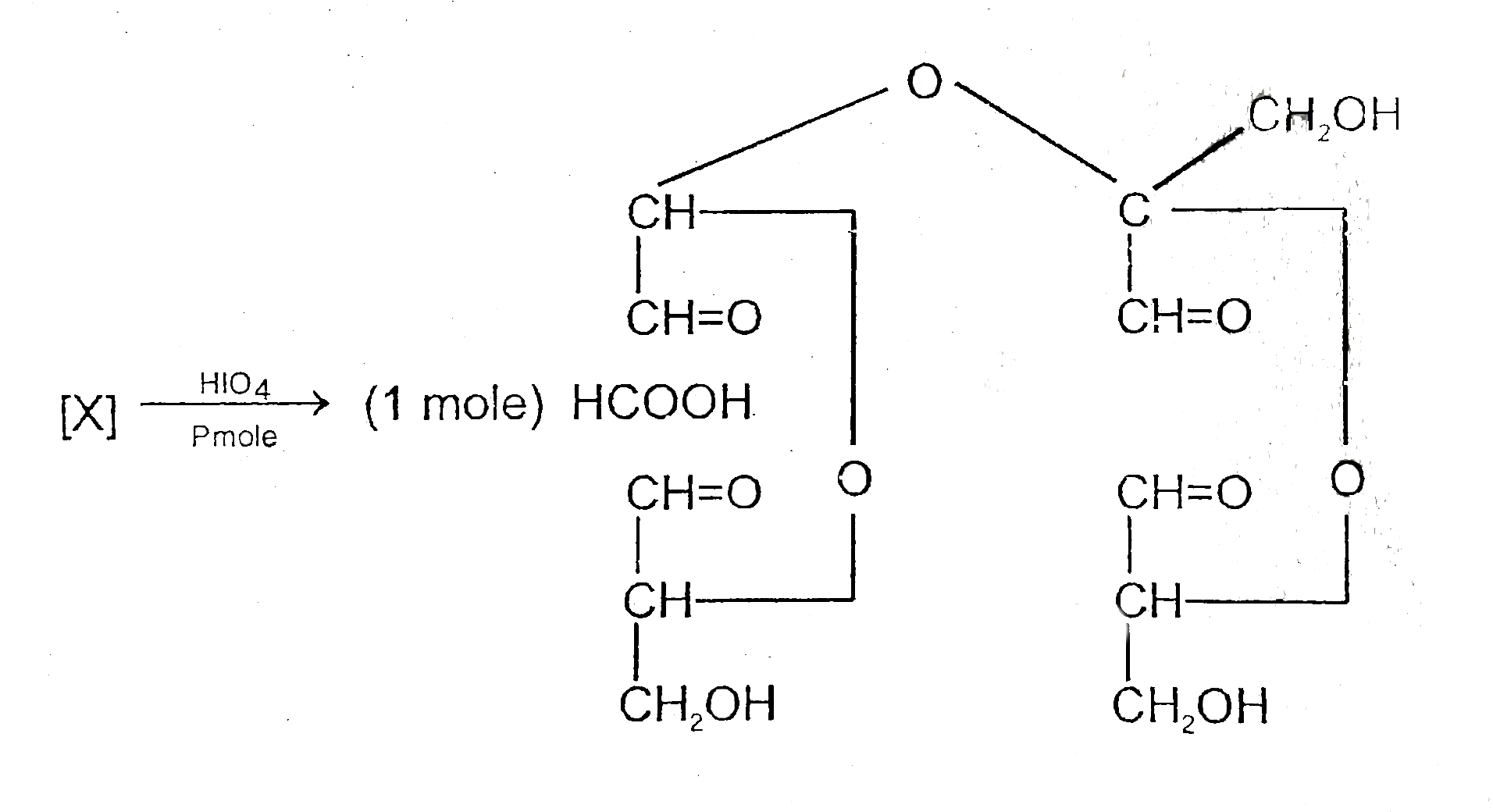
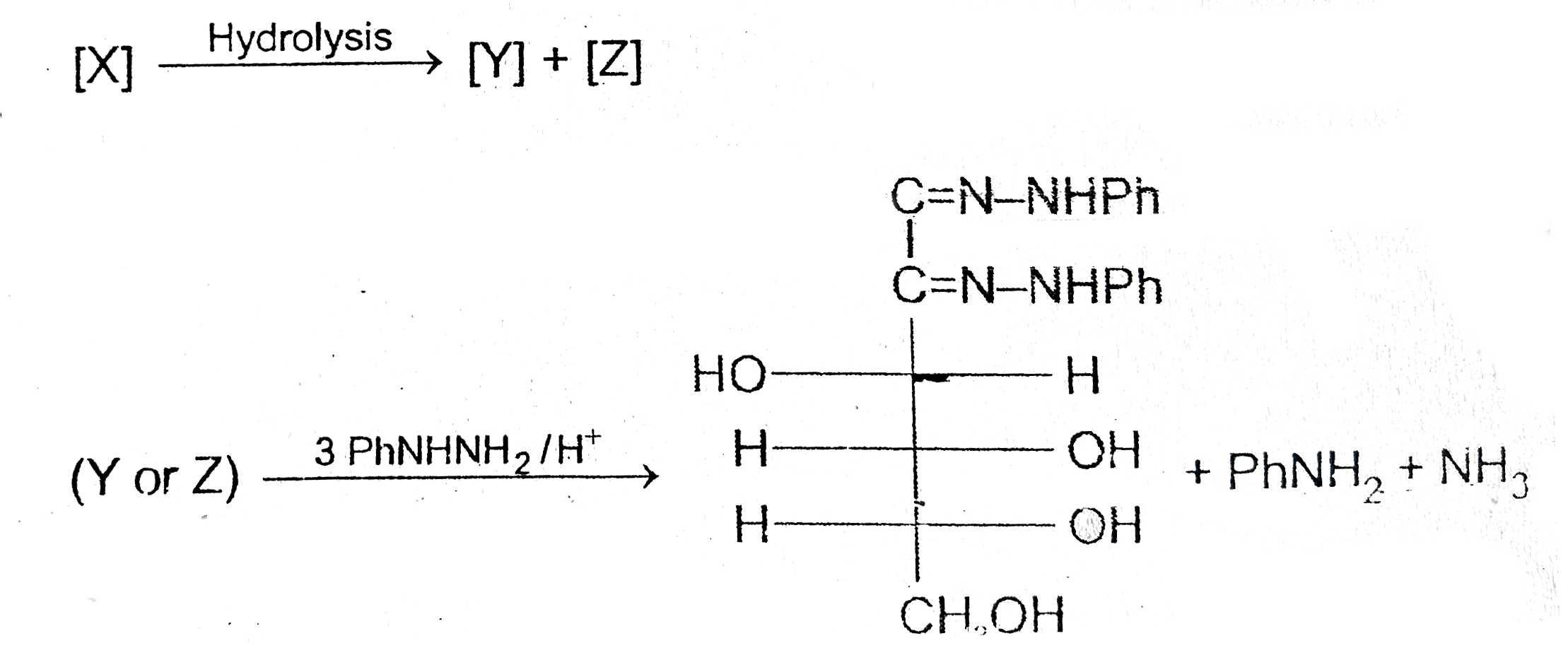
Number of moles (P) of HIO4 used per moles of compound X is :
View SolutionThe number of mole of oxalate ions oxidised by one mole of MnO_(4)^(-) is :
View Solutionअभिक्रिया A+B<⇒C+D में 4 मोल A को 4 मोल B के साथ अभिकृत करने पर उत्पाद C के 4 मोल बनते है, तो अभिक्रिया का साम्य सिथरांक है:
View SolutionOne mole of FeC_(2)O_(4) is oxidised by KMnO_(4) in acidic medium. Number of moles of KMnO_(4) used are
View SolutionNumber of moles of Oxygen needed to produce 4 moles of water on reacting with 4 moles of Hydrogen gas is ………………..
View SolutionHow many moles of hydrogen is required to produce 4 moles of ammonia?
View Solutionक्षारीय माध्यम में KIके एक मोल द्वारा अपचयित KMnO4 के मोलों की संख्या है
View Solutionफेरस ऑक्सेलेट के दो मोल के साथ क्रिया करने के लिए आवश्यक KMnO4 के मोलों की संख्या है?
View SolutionReduced by one mole KI in alkaline medium KMnO_(4) Number of moles:
View Solution

