If the concentration quotient of a reaction in greater than its equilibrium constant, then the reaction will proceed in the ______ direction.
To solve the question, "If the concentration quotient of a reaction is greater than its equilibrium constant, then the reaction will proceed in the ______ direction," we can follow these steps:
1. Understanding the Terms:
- The concentration quotient (Qc) is a ratio that represents the concentrations of products and reactants at any point in time for a given reaction.
- The equilibrium constant (Kc) is a specific value that represents the ratio of concentrations of products to reactants when the reaction is at equilibrium.
2. Comparing Qc and Kc:
- If Qc > Kc, it indicates that the concentration of products is greater than what it would be at equilibrium. This means the system is not at equilibrium.
3. Direction of the Reaction:
- When Qc > Kc, the reaction will shift to the left (or backward) to reduce the concentration of products and increase the concentration of reactants until equilibrium is reached.
4. Conclusion:
- Therefore, if the concentration quotient of a reaction is greater than its equilibrium constant, the reaction will proceed in the backward direction.
Final Answer:
The reaction will proceed in the backward direction.
---
- The concentration quotient (Qc) is a ratio that represents the concentrations of products and reactants at any point in time for a given reaction.
- The equilibrium constant (Kc) is a specific value that represents the ratio of concentrations of products to reactants when the reaction is at equilibrium.
- If Qc > Kc, it indicates that the concentration of products is greater than what it would be at equilibrium. This means the system is not at equilibrium.
- When Qc > Kc, the reaction will shift to the left (or backward) to reduce the concentration of products and increase the concentration of reactants until equilibrium is reached.
- Therefore, if the concentration quotient of a reaction is greater than its equilibrium constant, the reaction will proceed in the backward direction.
The reaction will proceed in the backward direction.
Topper's Solved these Questions
- EQUILIBRIUMBOOK - MODERN PUBLICATIONCHAPTER - EQUILIBRIUMEXERCISE - Revision Exercise (Assertion Reason )
15 Videos
- ENVIRONMENTAL POLLUTION BOOK - MODERN PUBLICATIONCHAPTER - ENVIRONMENTAL POLLUTION EXERCISE - UNIT PRACTICE TEST
15 Videos
- HALOALKANES AND HALOARENESBOOK - MODERN PUBLICATIONCHAPTER - HALOALKANES AND HALOARENESEXERCISE - UNIT PRACTICE TEST
12 Videos
Similar Questions
Knowledge Check
The reaction which proceeds in the forward direction is
AFe2O3+6HCl→2FeCl3+3H2OBNH3+H2O+NaCl→NH4Cl+NaOHCSnCl4+Hg2Cl2→SnCl2+2HgCl2D2CuI+I2+4H⊕→2Cu2++4KIThe reaction which proceeds in the forward direction is
AFe2O3+6HCl=2FeCl3+3H2OBNH3+H2O+NaCl=NH4Cl+NaOHCSnCl4+Hg2Cl2=SnCl2=SnCl2+2HgCl2D2CuI+I2+4K+=2Cu2++4KIThe unit for the equilibrium constant of the reaction
A[mole/lit]−B[mole/lit]−2CMole/litD[mole/lit]2
The equilibrium constant for the following reaction is
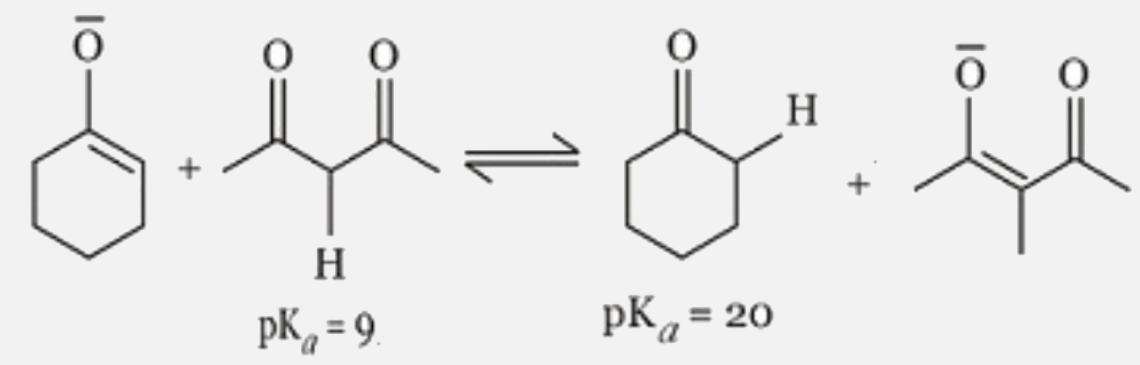
View SolutionThe equilibrium constant for the following reaction is

View SolutionEquilibrium constant of a reaction is related to
View SolutionEquilibrium constant of a reaction is elated to :
View SolutionThe equilibrium constant of the following reaction is
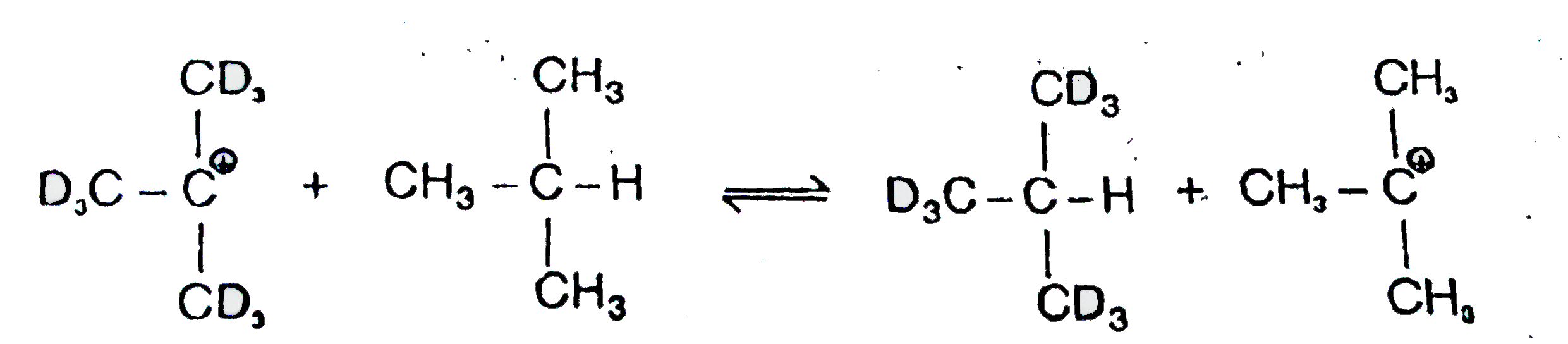
View Solution

