Arrange the following set of compounds in the order of decreasing reactivity with an electrophile. Give reason.


The +R effect of −OCH3 group is more than that of-CI, whereas -NO, group has -R effect. Therefore, reactivity of the substituted benzene rings is
Topper's Solved these Questions
- HYDROCARBONSBook:MODERN PUBLICATIONChapter:HYDROCARBONSExercise:NCERT FILE (NCERT EXEMPLAR PROBLEMS) (MATCH TYPE QUESTIONS)
Explore 4 Videos
- HYDROCARBONSBook:MODERN PUBLICATIONChapter:HYDROCARBONSExercise:NCERT FILE (NCERT EXEMPLAR PROBLEMS) (Assertion and Reason Type Questions)
Explore 3 Videos
- HYDROCARBONSBook:MODERN PUBLICATIONChapter:HYDROCARBONSExercise:NCERT FILE (NCERT EXEMPLAR PROBLEMS) (Multiple Choice Questions (Type-II))
Explore 9 Videos
- HALOALKANES AND HALOARENESBook:MODERN PUBLICATIONChapter:HALOALKANES AND HALOARENESExercise:UNIT PRACTICE TEST
Explore 12 Videos
- HYDROGENBook:MODERN PUBLICATIONChapter:HYDROGENExercise:UNIT PRACTICE TEST (for Board Examination)
Explore 9 Videos
Similar Questions
Match the reagent from Column I which on reaction with CH3−CH=CH2, gives some product given in Column II as per the codes given below:

View SolutionAssertion (A) : Toluene on Friedel-Crafts Methylation gives o - and p-xylene.
Reason ( R) : CH3 group bonded to benzene ring increases density at o - and p- position.
View SolutionSome oxidation reactions of methane are given below. Which of them is/ are controlled oxidation reactions?
View SolutionThe hydrolysis of 2-bromo-3-methylbutane by SN1 mechanism gives mainly:
View SolutionDecomposition of hydrogen peroxide can be prevented by
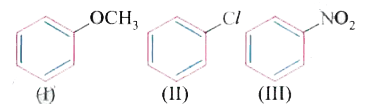
View SolutionWhich of the following compounds are not arranged in order of decreasing reactivity towards electrophilic substitution
View SolutionArrange the following in decreasing order of reactivity towards EAS (electrophilic aromatic substitution)
(a)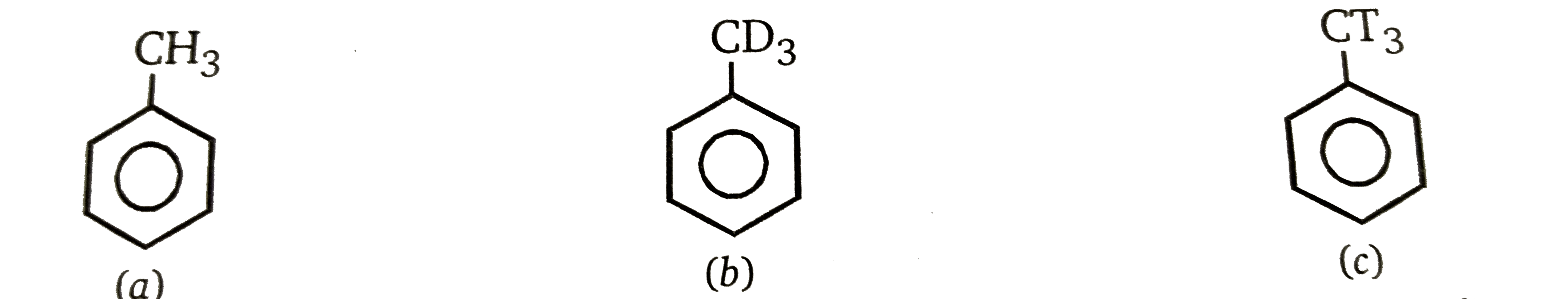
View SolutionArrange the following set of compounds in the order of their decreasing relative reactivity with an electrophile . Give reason.
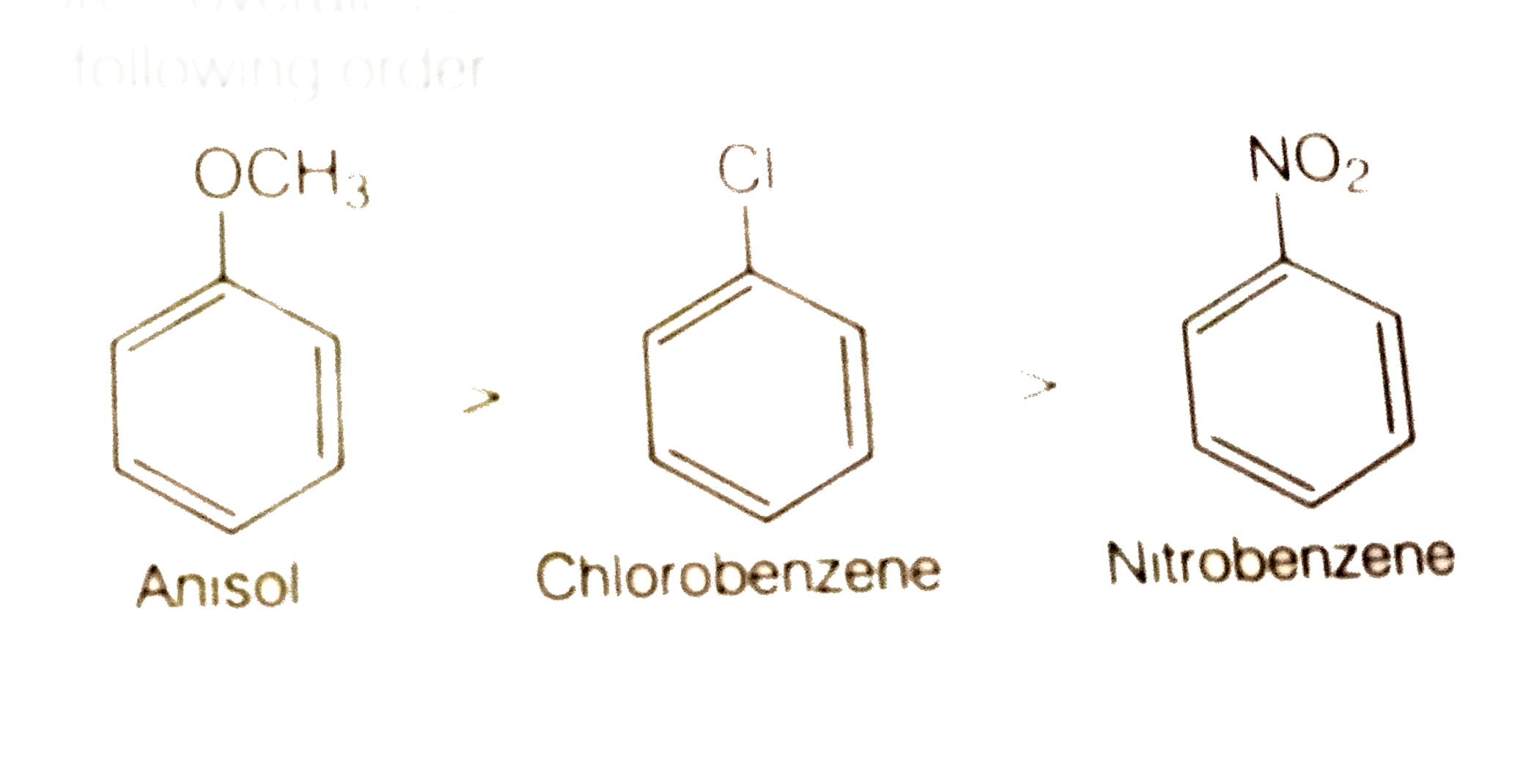
View SolutionArrange the following in decreasing order of their reactivity with an electrophile.
View SolutionArrange the following in decreasing order of reactivity towards electrophilic substitution:
View SolutionWhich of the following compounds are arranged in order of decreasing reactivity towards electrophilic substitution
View SolutionArrange the following set of compounds in order of their decreasing relative reactivity with an electrophile, E^+ . Chlorobenzene,2,4- dinitrochlorobenzene, p-nitrochlorobenzene
View SolutionArrange the following in the decreasing order of reactivity towards electrophilic substitution is
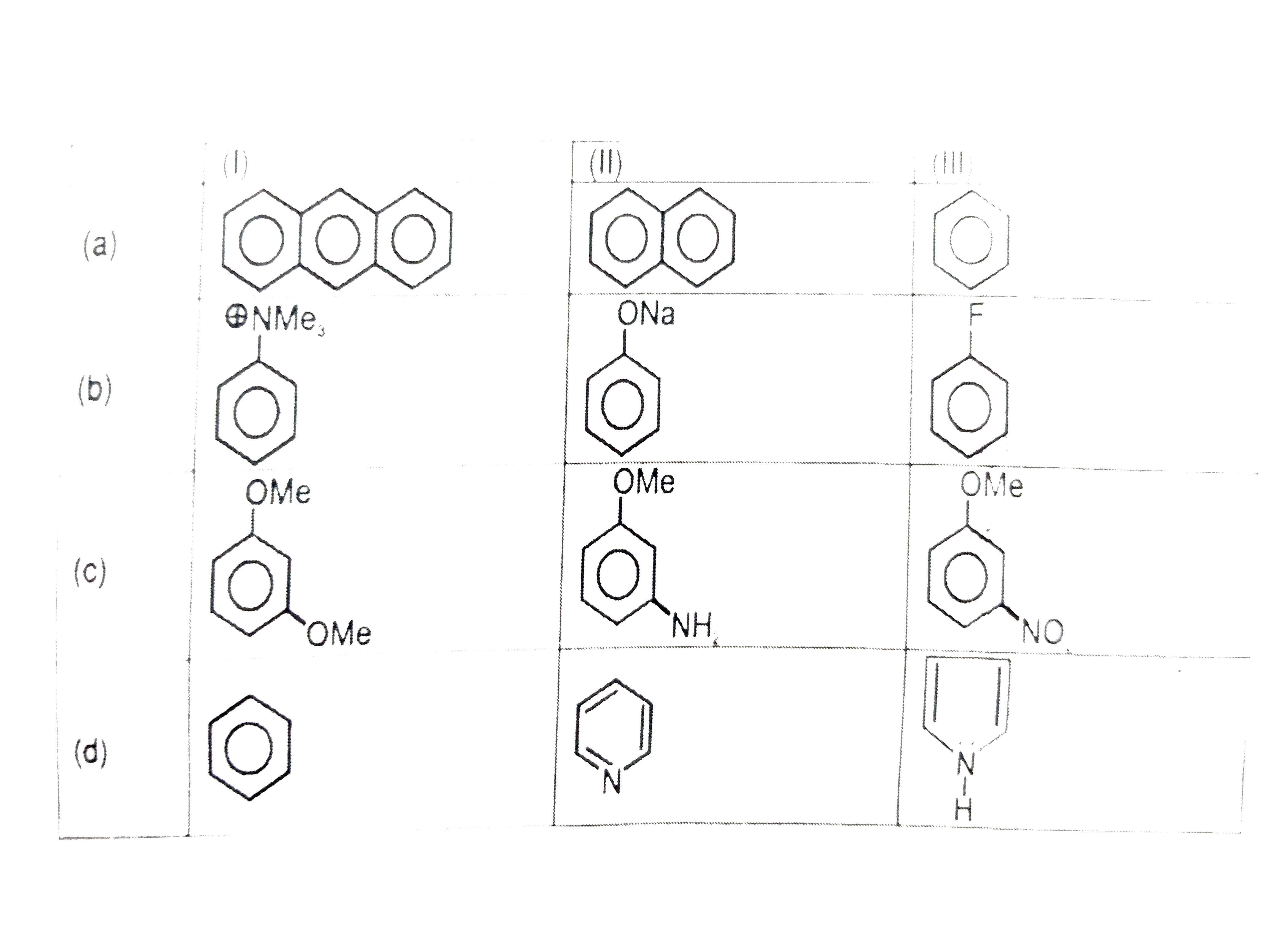
View SolutionArrange the following set of compounds in the order of their decreasing relative reactivity with an electrophile
View SolutionArrange the following set of compounds in the order of their decreasing relative reactivity with an electrophile . Give reason.

View SolutionArrange the following set of compounds in order of their decreasing relative reactivity with an electrophile, E+ .
Chlorobenzene, 2, 4-dinitrochlorobenzene and p-nitrochlorobenzene.
View Solution

