Account for the variation of acidic character with increases in the oxidation state of central atom
Video Solution
Text Solution
(i) effect of electronegative atom on the O−H bond strength
(ii) effect of oxidation state of the central atom on the position of electron cloud present between oxygen atom and the central atom
(iii) relation between the position of the above electron cloud and the charge separation between hydrogen and oxygen atom of the hydroxylradical attached to the central atom
(iv) relation between the above charge separation and ease of formation of hydrogen ion
(v) relation between ease of formation of hydrogen ion with the acidic character of the respective acids
|
Similar Questions
Knowledge Check
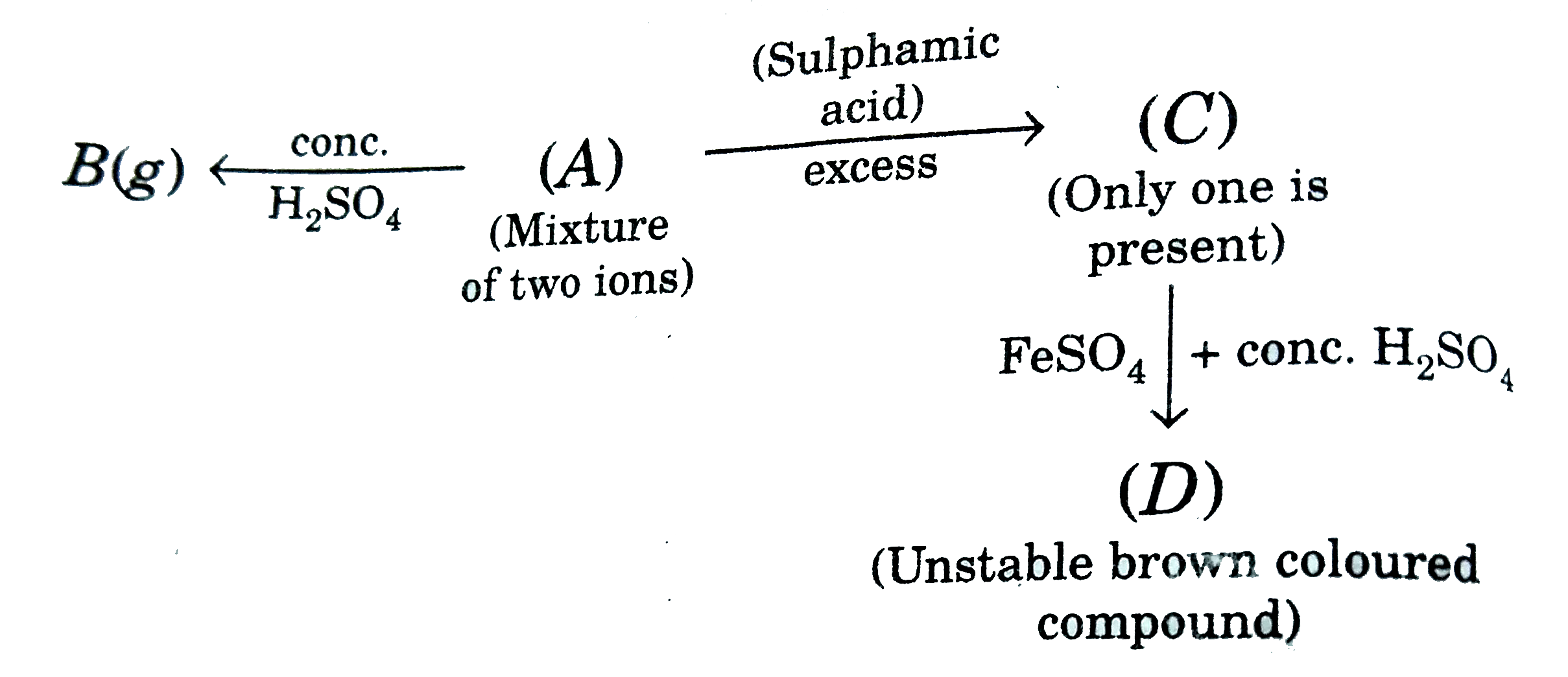
What is oxidation state of central atom of (D)?A+3B+2C+1DZeroThe oxidation state of O-atom is +2 in
AOF2BO2F2CH2ODH2O2The species with an atom in +6 oxidation state is
AMnO−4BCr(CN)3−6CNiF2−6DCrO2Cl2
If the oxidation state of central atom in BH3 is +3, then the oxidation states of the central atom in SiH4andCH4 are, respectively,
View SolutionWhich of the following is a decreasing order of oxidation states of the central atoms?
View SolutionIncreasing order of acidic character would be
View SolutionWhich of the following arrangements represent increaseing oxidation number of the central atom?
View SolutionWhich of the following arrangements represent increaseing oxidation number of the central atom?
View Solution
