What property of molecules of real gases is indicated by Van der Waals constant 'a'? (ii) has larger molecular size ?
To answer the question regarding the Van der Waals constant 'a', we can break down the solution into clear steps:
1. Understanding Van der Waals Equation:
The Van der Waals equation is given by:
(P+an2V2)(V−nb)=nRT
Here, P is the pressure, V is the volume, n is the number of moles, R is the universal gas constant, T is the temperature, and a and b are the Van der Waals constants.
2. Identifying the Role of 'a':
The constant 'a' in the Van der Waals equation accounts for the attractive forces between the gas molecules. It corrects the pressure in the equation to account for these intermolecular attractions.
3. Intermolecular Forces:
The value of 'a' indicates the strength of the intermolecular forces. A larger value of 'a' suggests stronger attractive forces between the molecules, which is characteristic of real gases compared to ideal gases, where such forces are neglected.
4. Conclusion:
Therefore, the property of molecules of real gases indicated by the Van der Waals constant 'a' is the intermolecular attraction. This means that the larger the value of 'a', the stronger the intermolecular forces present in the gas.
5. Addressing the Second Part:
The second part of the question asks if 'a' indicates larger molecular size. While 'a' is related to intermolecular forces, the constant 'b' in the Van der Waals equation is the one that indicates the volume occupied by the gas molecules, which is related to their size. Thus, 'a' does not directly indicate larger molecular size.
Final Answer:
(i) The property of molecules of real gases indicated by the Van der Waals constant 'a' is intermolecular attraction.
(ii) No, 'a' does not indicate larger molecular size; that is indicated by the constant 'b'.
The Van der Waals equation is given by:
(P+an2V2)(V−nb)=nRT
Here, P is the pressure, V is the volume, n is the number of moles, R is the universal gas constant, T is the temperature, and a and b are the Van der Waals constants.
The constant 'a' in the Van der Waals equation accounts for the attractive forces between the gas molecules. It corrects the pressure in the equation to account for these intermolecular attractions.
The value of 'a' indicates the strength of the intermolecular forces. A larger value of 'a' suggests stronger attractive forces between the molecules, which is characteristic of real gases compared to ideal gases, where such forces are neglected.
Therefore, the property of molecules of real gases indicated by the Van der Waals constant 'a' is the intermolecular attraction. This means that the larger the value of 'a', the stronger the intermolecular forces present in the gas.
The second part of the question asks if 'a' indicates larger molecular size. While 'a' is related to intermolecular forces, the constant 'b' in the Van der Waals equation is the one that indicates the volume occupied by the gas molecules, which is related to their size. Thus, 'a' does not directly indicate larger molecular size.
(i) The property of molecules of real gases indicated by the Van der Waals constant 'a' is intermolecular attraction.
(ii) No, 'a' does not indicate larger molecular size; that is indicated by the constant 'b'.
Topper's Solved these Questions
- STATES OF MATTER : GASES AND LIQUIDSBOOK - MODERN PUBLICATIONCHAPTER - STATES OF MATTER : GASES AND LIQUIDSEXERCISE - NCERT FILE (NCERT)(Textbook Exercises)
23 Videos
- STATES OF MATTER : GASES AND LIQUIDSBOOK - MODERN PUBLICATIONCHAPTER - STATES OF MATTER : GASES AND LIQUIDSEXERCISE - NCERT FILE NCERT (EXemplar Problems) (Multiple Choice Questions (Type-I))
24 Videos
- STATES OF MATTER : GASES AND LIQUIDSBOOK - MODERN PUBLICATIONCHAPTER - STATES OF MATTER : GASES AND LIQUIDSEXERCISE - Conceptual Questions (1)
17 Videos
- SOME BASIC CONCEPTS OF CHEMISTRY BOOK - MODERN PUBLICATIONCHAPTER - SOME BASIC CONCEPTS OF CHEMISTRY EXERCISE - COMPETITION FILE (INTEGER TYPE AND NUMERICAL VALUE TYPE QUESTIONS)
10 Videos
- STRUCTURE OF ATOMBOOK - MODERN PUBLICATIONCHAPTER - STRUCTURE OF ATOMEXERCISE - Unit Practice Test
13 Videos
Similar Questions
What will be the minimum pressure required to compress 500 dm3 of air at 1 bar to 200 dm3 at 30∘C ?
View SolutionA person living in shimla observd that cooking without using pressure cooker takes more time. The reason for this observation is that at high altitude
View SolutionHow is the pressure of a gas related to its density at a particular temperature ?
View Solution1.420 g of anhydrous ZnSO4 was left in moist air. After a few days its weight was found to be 2.528 g. How many water molecules are present in its hydrated salt formula (molar mass of ZnSO4 = 161.5)?
View SolutionMaximum number of electrons in a sub-shell of an atom is determined by the following.
View SolutionExplanation Of The Behaviour Of Real Gases By Van Der Waals Equation
View SolutionWhat is the value of b (van der Waals constant) if the diameter of a molecule is 2.0
View SolutionThe van der Waals constant for gases A , B , and C are as follows Answer the following: Which gas has the largest molecular volume?
View SolutionArrange the van der Waals constant for the gases .
View Solutionvan der Waals constant b in corrected equation for real gases represents
View SolutionFor real gases (van der waals ' gas )
View SolutionIn van der waals equation, ______is a measure of the effective size of the gas molecules.
View SolutionThe van der Waal constants of two gases are as follows :
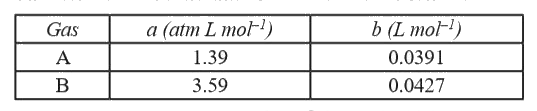
Which of them is more easily liquefiable and which has greater molecular size?
View SolutionIn van der Waals equation, the constant of accounts for what property of molecules of real gases ?
View Solutionvan der Waals constant b in corrected equation for real gases represents
View Solution

