LiH is more stable than NaH. Explain.
To explain why lithium hydride (LiH) is more stable than sodium hydride (NaH), we can analyze the factors affecting their stability, particularly focusing on lattice energy and ionic size.
1. Understanding the Ionic Compounds:
- LiH is composed of lithium ions (Li⁺) and hydride ions (H⁻).
- NaH is composed of sodium ions (Na⁺) and hydride ions (H⁻).
2. Comparison of Ionic Sizes:
- Lithium ion (Li⁺) is smaller in size compared to sodium ion (Na⁺).
- The smaller size of Li⁺ leads to a higher charge density, which means it can exert a stronger electrostatic attraction on the hydride ion (H⁻).
3. Lattice Energy:
- Lattice energy is the energy released when gaseous ions combine to form an ionic solid. It is directly related to the size of the ions involved.
- Since Li⁺ is smaller than Na⁺, the lattice energy of LiH is greater than that of NaH. This is because the smaller Li⁺ can get closer to the H⁻ ion, resulting in a stronger attraction.
4. Stability of the Compounds:
- A higher lattice energy implies that more energy is required to break the ionic bonds in LiH compared to NaH.
- Therefore, the stronger the ionic bond (due to higher lattice energy), the more stable the compound is.
5. Conclusion:
- As a result, LiH is more stable than NaH because the smaller size of Li⁺ leads to greater lattice energy, making the ionic bond in LiH stronger and more difficult to break.
Summary:
LiH is more stable than NaH due to the smaller size of the lithium ion, which results in higher lattice energy and stronger ionic bonds compared to sodium hydride.
- LiH is composed of lithium ions (Li⁺) and hydride ions (H⁻).
- NaH is composed of sodium ions (Na⁺) and hydride ions (H⁻).
- Lithium ion (Li⁺) is smaller in size compared to sodium ion (Na⁺).
- The smaller size of Li⁺ leads to a higher charge density, which means it can exert a stronger electrostatic attraction on the hydride ion (H⁻).
- Lattice energy is the energy released when gaseous ions combine to form an ionic solid. It is directly related to the size of the ions involved.
- Since Li⁺ is smaller than Na⁺, the lattice energy of LiH is greater than that of NaH. This is because the smaller Li⁺ can get closer to the H⁻ ion, resulting in a stronger attraction.
- A higher lattice energy implies that more energy is required to break the ionic bonds in LiH compared to NaH.
- Therefore, the stronger the ionic bond (due to higher lattice energy), the more stable the compound is.
- As a result, LiH is more stable than NaH because the smaller size of Li⁺ leads to greater lattice energy, making the ionic bond in LiH stronger and more difficult to break.
LiH is more stable than NaH due to the smaller size of the lithium ion, which results in higher lattice energy and stronger ionic bonds compared to sodium hydride.
Similar Questions
Knowledge Check
Aniline is more stable than anilinium ion because of
Adifferent chargesBdifferent π bondsCdifferent resonating structureDdifferent N atomsIn which of the following pairs A is more stable than B ?
A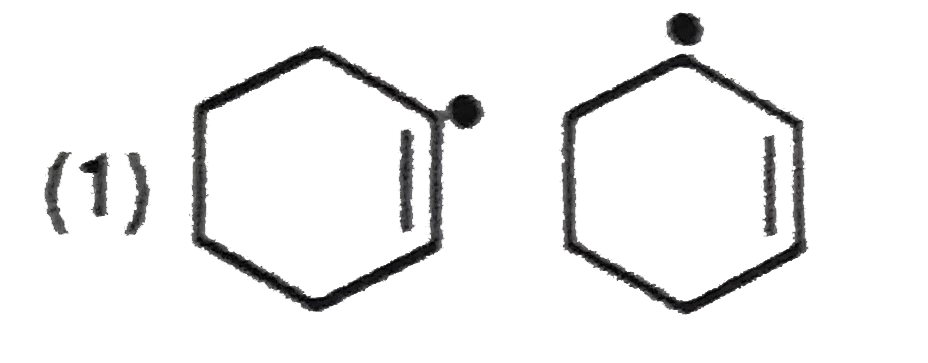 B
B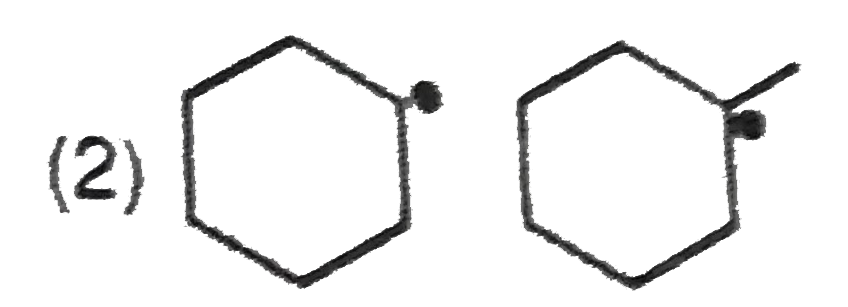 C
C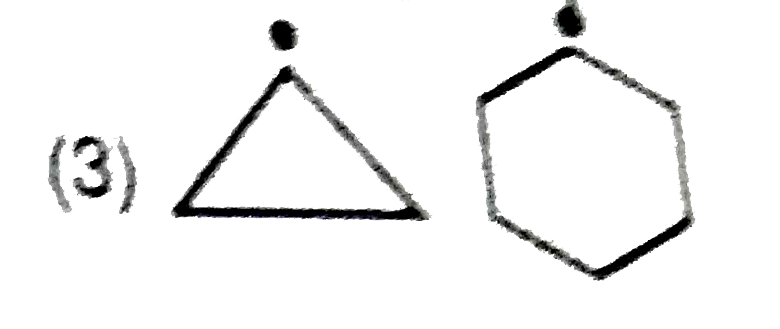 DPh3C⋅,(CH3)C⋅
DPh3C⋅,(CH3)C⋅In which of the following pairs A is more stable than B?
A B
B C
C D
D
Manganese in the +2 oxidation state is more stable than +3 oxidation state where as iron is stable at +3 oxidation state than +2 oxidation state. Explain why
View SolutionIn which of the following pairs A is more stable than B ?
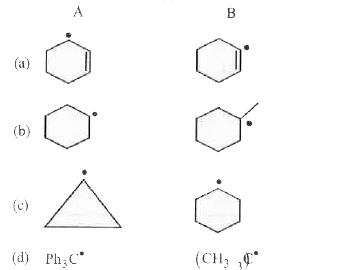
View SolutionTlCl3 is more stable than TlCl.
+1 oxidation state of thallium us more stable than +3.
View SolutionIn which of the following 2nd anion is more stable than first?
View SolutionA : Cyclohexane is more stable than cyclopentane.
R : According to Baeyer strain theory angle strain in cyclohexane is more than cyclopentane.
View Solution

