Why [CuCl4]2− exist but [Cul4]2− does not exist.
I− is a better reducing agent than Cl− and reduces Cu2+ and itself changes to I2.
Topper's Solved these Questions
- CHEMICAL ENERGETICS BOOK - FIITJEECHAPTER - CHEMICAL ENERGETICS EXERCISE - ASSIGNMENT PROBLEMS (SECTION (I) JEE MAINS : SINGLE CORRECT OPTION)
92 Videos
- CHEMICAL ENERGETICS BOOK - FIITJEECHAPTER - CHEMICAL ENERGETICS EXERCISE - ASSIGNMENT PROBLEMS (SECTION (II) JEE ADVANCED WITH SINGLE CORRECT CHOICE)
84 Videos
- CARBOXYLIC ACIDS AND ITS DERIVATIVESBOOK - FIITJEECHAPTER - CARBOXYLIC ACIDS AND ITS DERIVATIVESEXERCISE - SINGLE INTEGER ANSWER TYPE QUESTIONS
1 Video
- CHEMICAL EQUILIBRIUMBOOK - FIITJEECHAPTER - CHEMICAL EQUILIBRIUMEXERCISE - MATCHING TYPE QUESTIONS
3 Videos
Similar Questions
Which of the following metal can be obtained by the thermal decomposition of its oxide?
View Solution[Co(NH3)5Br]SO4 and conc. [Co(NH3)5SO4]Br are example of which type of isomerism?
View SolutionFind the number of geometrical isomers of the given compound .
C6H5−CH=CH−CH−CH=CH−CH=CH−COOH
View Solution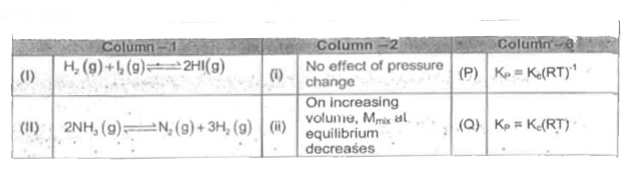

Which of the following combination is correct ?
View SolutionWhy [BF6]2− ion does not exist?
View SolutionCIF2 exists but FCl3 does not. Why?
View SolutionWhy does a molecule of Ne2 fail to exist ?
View SolutionKHF2 exists while KHCl2 does not. Explain.
View Solution[CuCl4]2− स्थायी है, परन्तु [CuI4]2− नहीं, क्यों ?
View Solution(CuCI4)−2 का अस्तित्व होता है जबकि (CuI4)2− का अस्तित्व नहीं होता । क्यों ?
View Solutionआणविक ऑर्बिटल सिद्धान्त के आधार पर स्प्ष्ट कीजिए , क्यों He2 का अस्तित्व नहीं होता है जबकि He+2 का अस्तित्व होता है ?
View SolutionStatement-1: The species [CuCl_(4)]^(2-) exists but [CUI_(4)]^(2-) does not. Statement-2: [NiCl_(2)(PPh_(3))_(2)] have tetrahedral geometry.
View SolutionStatement-1: The species [CuCl_(4)]^(2-) exists but [CUI_(4)]^(2-) does not. Statement-2: [NiCl_(2)(PPh_(3))_(2)] have tetrahedral geometry.
View SolutionOF2 exists but OF4 does not because:
View Solution[CuCl_4) ^(2-) exists while [CuI_4) ^(2-) does not exist why?
View Solution

