Given,
NH3(g)+3Cl2(g)NCl3(g)+3HCl,−ΔH1
N2(g)+Cl2(g)→2NH3(g),−ΔH2
H2(g)+Cl2(g)→2HClg,−ΔH3
The heat of formation of NCl3(g) in the terms of ΔH1,ΔH2 and ΔH3 is
ΔrH=−ΔH1+ΔH22−32ΔH3
Δ1H=ΔH1+ΔH22−32ΔH2
ΔH=ΔH1−ΔH22−32ΔH2
none of these
The correct Answer is:D
Topper's Solved these Questions
- CHEMICAL ENERGETICS Book:FIITJEEChapter:CHEMICAL ENERGETICS Exercise:ASSIGNMENT PROBLEMS (SECTION (II) JEE ADVANCED WITH SINGLE CORRECT CHOICE)
Explore 84 Videos
- CHEMICAL ENERGETICS Book:FIITJEEChapter:CHEMICAL ENERGETICS Exercise:MORE THAN ONE CORRECT OPTION
Explore 32 Videos
- CHEMICAL ENERGETICS Book:FIITJEEChapter:CHEMICAL ENERGETICS Exercise:NUMERICAL BASED QUESTIONS
Explore 2 Videos
- CARBOXYLIC ACIDS AND ITS DERIVATIVESBook:FIITJEEChapter:CARBOXYLIC ACIDS AND ITS DERIVATIVESExercise:SINGLE INTEGER ANSWER TYPE QUESTIONS
Explore 1 Video
- CHEMICAL EQUILIBRIUMBook:FIITJEEChapter:CHEMICAL EQUILIBRIUMExercise:MATCHING TYPE QUESTIONS
Explore 3 Videos
Similar Questions
[Co(NH3)5Br]SO4 and conc. [Co(NH3)5SO4]Br are example of which type of isomerism?
View SolutionWhich of the following statements is/are true about an azeotropic mixture?
View SolutionCalculate the ratio of Z in bcc of fcc?
View SolutionFind the number of geometrical isomers of the given compound .
C6H5−CH=CH−CH−CH=CH−CH=CH−COOH
View Solution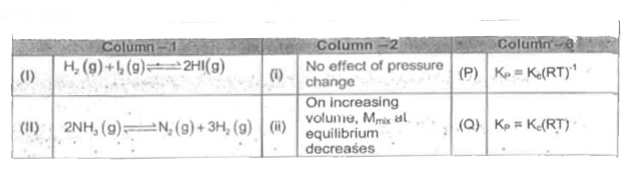

Which of the following combination is correct ?
View SolutionGiven a. NH_(3)(g)+3CI(g)rarr NCI_(3)(g),3HCI(g),DeltaH_(1) b. N_(2)(g)+3H_(2)(g)rarr 2NH_(3)(g),DeltaH_(2) c. H_(2)(g)+CI_(2)(g)rarr 2HCI(g),DeltaH_(3) Express the enthalpy of formation of NCI_(3)(g)(Delta_(f)H^(Theta)) in terms of DeltaH_(1), DeltaH_(2) ,and DeltaH_(3) .
View SolutionNH_(3)(g)+3C1_(2)(g)rarrNCL_(3)(g)+3HC1(g)DeltaH_(1) N_(2)(g)+3H_(2)(g)rarr2NH_(3),DeltaH_(2) H_(2)(g)+CL_(2)(g)rarr2HC1(g),DeltaH_(1) The heat of fomation of NC1_(2)(g) in the terms of DeltaH_(1) , DeltaH_(2) and DeltaH_(3) is
View SolutionNH_(3)(g)+3Cl_(2)toNCl_(3)(g)+3HCl(g),DeltaH_(1) N_(2)(g)+3H_(2)(g)to2NH_(3)(g),DeltaH_(2) H_(2)(g)+Cl_(2)(g)to 2HCl(g),DeltaH_(3) The heat of formation of NCl_(3) (g) in the terms of DeltaH_(1),DeltaH_(2),DeltaH_(3) is :
View SolutionUse the given standard enthalpies of formation (in kJ/mol) to determine the enthalpy of reaction of the following reaction : NH_(3)(g)+3F_(2)(g)rarrNF_(3)+3HF(g) DeltaH_(f)^(@)(NH_(3),g)=-46.2, " "DeltaH_(f)^(@)(NF_(3),g)=-113.0 ," "DeltaH_(f)^(@)(HF, g)=-269.0
View SolutionGiven : (i) NH_(3) (g) + 3Cl_(2)(g) rarr NCl_(3)(g)+3HCl(g) , Delta H_(1) (ii) N_(2)(g) + 3H_(2)(g) rarr 2NH_(3)(g) , Delta H_(2) (iii) H_(2)(g)+Cl_(2)(g) rarr2HCl(g) , Delta H_(3) Express the enthalpy of formation of NCl_(3)(g)(Delta H_(f)) in terms of DeltaH_(1), Delta H_(2) and Delta H_(3) :
View SolutionGiven NH_(3)(g)+3Cl_(2)(g)toNCl_(3)(g)+3HCl(g)+x_(1) N_(2)(g)+3H_(2)(g)to2NH_(3)(g)+x_(2) H_(2)(g)+Cl_(2)(g)to2HCl(g)-x_(3) The heat of formation of NCl_(3)(g)
View SolutionFor the reaction
3N2O(g)+2NH3(g)→4N2(g)+3H2O(g),ΔH∘=−879.6kJ
If ΔH∘f[NH3(g)]=−45.9kJ mol−1,
ΔH∘f[H2O(g)]=−241.8kJ mol−1
Then ΔH∘f[N2O(g)] will be:
View SolutionNH3(g)+3Cl2(g)→NCl3(g)+3HCl(g), ΔH1
N2(g)+3H2(g)→2NH3(g), ΔH2
H2(g)+Cl2(g)→2HCl(g), ΔH3
The heat of formation of NCl3 in the terms of ΔH1,ΔH2andΔH3 is
View SolutionConsider the following reactions
(i) A(s)→A(g):ΔH1
A(g)→A+(g):ΔH2
A+(g)→A2+(g):ΔH3
then according to the given information, select the correct statement(s)
View SolutionGiven, NH_(3(g))+3Cl_(2(g))iffNCl_(3(g))+3HCl_((g)),-DeltaH_(1) N_(2(g))+3H_(2(g))iff2NH_(3(g)),-DeltaH_(2) H_(2(g))+Cl_(2(g))iff2HCl_((g)),DeltaH_(3) The heat of formation of NCl_(3(g)) in the terms of DeltaH_(1), DeltaH_(2) and DeltaH_(3) is
View Solution

