Arrange the following compounds in increasing order of their acid strength:
Propan-1-ol: 2, 4, 6-trinitrophenol, nitrophenol, 3,5-dinitrophenol: phenol: 4-methylphenol.M.C.E.R.L Ans. Increasing order of acid strength is : Propan-1-ol, 4-methylphenol, phenol, 3-nitrophenol, 3, 5-dinitrophenol, 2, 4, 6-trinitrophenol.
Topper's Solved these Questions
- ALCOHOLS, PHENOLS AND ETHERSBook:MODERN PUBLICATIONChapter:ALCOHOLS, PHENOLS AND ETHERSExercise:CONCEPTUAL QUESTIONS 2
Explore 15 Videos
- ALCOHOLS, PHENOLS AND ETHERSBook:MODERN PUBLICATIONChapter:ALCOHOLS, PHENOLS AND ETHERSExercise:NCERT (IN-TEXT EXERCISES)
Explore 12 Videos
- ALCOHOLS, PHENOLS AND ETHERSBook:MODERN PUBLICATIONChapter:ALCOHOLS, PHENOLS AND ETHERSExercise:PRACTICE PROBLEM
Explore 26 Videos
- ALDEHYDES ,KETONES AND CARBOXYLIC ACIDSBook:MODERN PUBLICATIONChapter:ALDEHYDES ,KETONES AND CARBOXYLIC ACIDSExercise:UNIT PRACTICE TEST
Explore 13 Videos
Similar Questions
(a) Give chemical tests to distinguish between the following pairs of compounds:
(i) Ethanal and Propanone.
(ii) Pentan-2-one and Pentan-3-one.
(b) Arrange the following compounds in increasing order of their acid strength: Benzoic acid, 4- Nitrobenzoic acid, 3,4-Dinitrobenzoic acid,
4- Methoxybenzoic acid.
View Solution(a) Give chemical tests to distinguish between the following pairs of compounds:
(i) Ethanal and Propanone.
(ii) Pentan-2-one and Pentan-3-one.
(b) Arrange the following compounds in increasing order of their acid strength: Benzoic acid, 4- Nitrobenzoic acid, 3,4-Dinitrobenzoic acid,
4- Methoxybenzoic acid.
View Solution
Knowledge Check
Arrange the following compounds in increasing order of their acidic strength:
(i) m-nitrophenol (ii) m-cresol
(iii) phenol (iv) m-chlorophenolAii<iii<iv<iBiii<ii<I<ivCii<iv<iii<iDii<iii<I<ivArrange the following compounds in increasing order of their acidic strength.
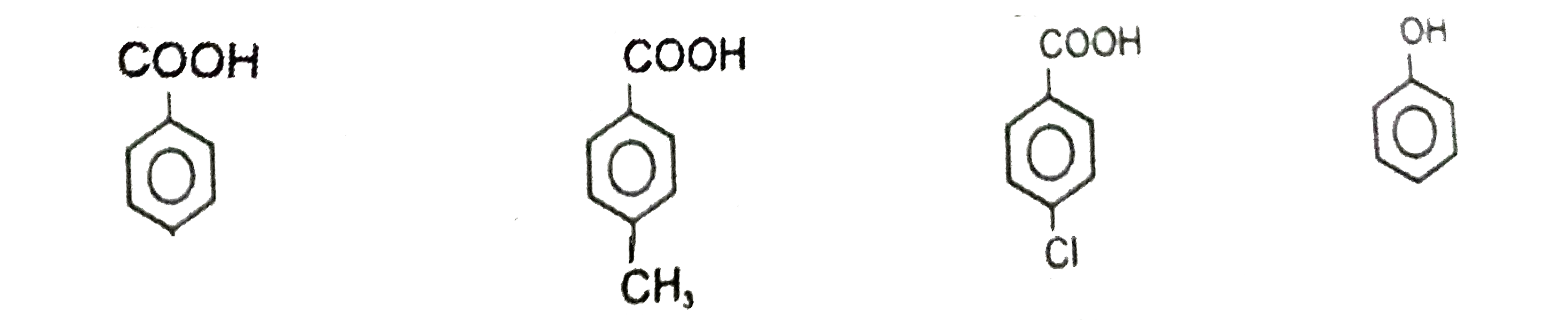 AI<II<III<IV<VBIV<I<III<II<VCIII<II<I<IV<VDII<I<III<IV<V
AI<II<III<IV<VBIV<I<III<II<VCIII<II<I<IV<VDII<I<III<IV<V
Arrange the following compounds in increasing order of their acidic strength :
Propan -1-ol 2, 4, 6-trinitrophenol, 3-nitrophenol, 3, 5-dinitrophenol, phenol, 4-methylphenol.
View SolutionArrange the following compounds in increasing order of their acidic strength.
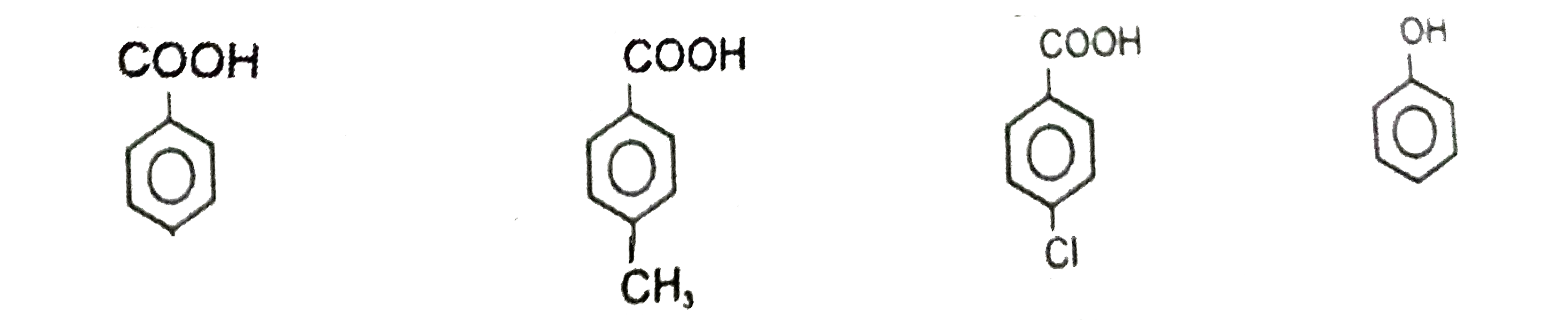
View SolutionArrange the following compounds in an increasing order of their acid strengths :
(CH3)2CHCOOH,CH3CH2CH(Br)COOH,CH3CH(Br)CH2COOH
View SolutionArrange the following compounds in an increasing order of their acid strengths :
(CH3)2CHCOOH,CH3CH2CH(Br)COOH,CH3CH(Br)CH2COOH
View SolutionArrange the following compounds in the increasing order of their acid strengths :
4-nitrophenol, phenol, 2,4,6-trinitrophenol.
View Solution(a) Arrange the following compounds in the increasing order of their acid strength :
p - cresol, p - nitrophenol, phenol
(b) Write teh mechanism (using curved arrow notation) of the following reaction :
CH2=CH2H3O+−−−→CH3−CH+2+H2O
View Solution

