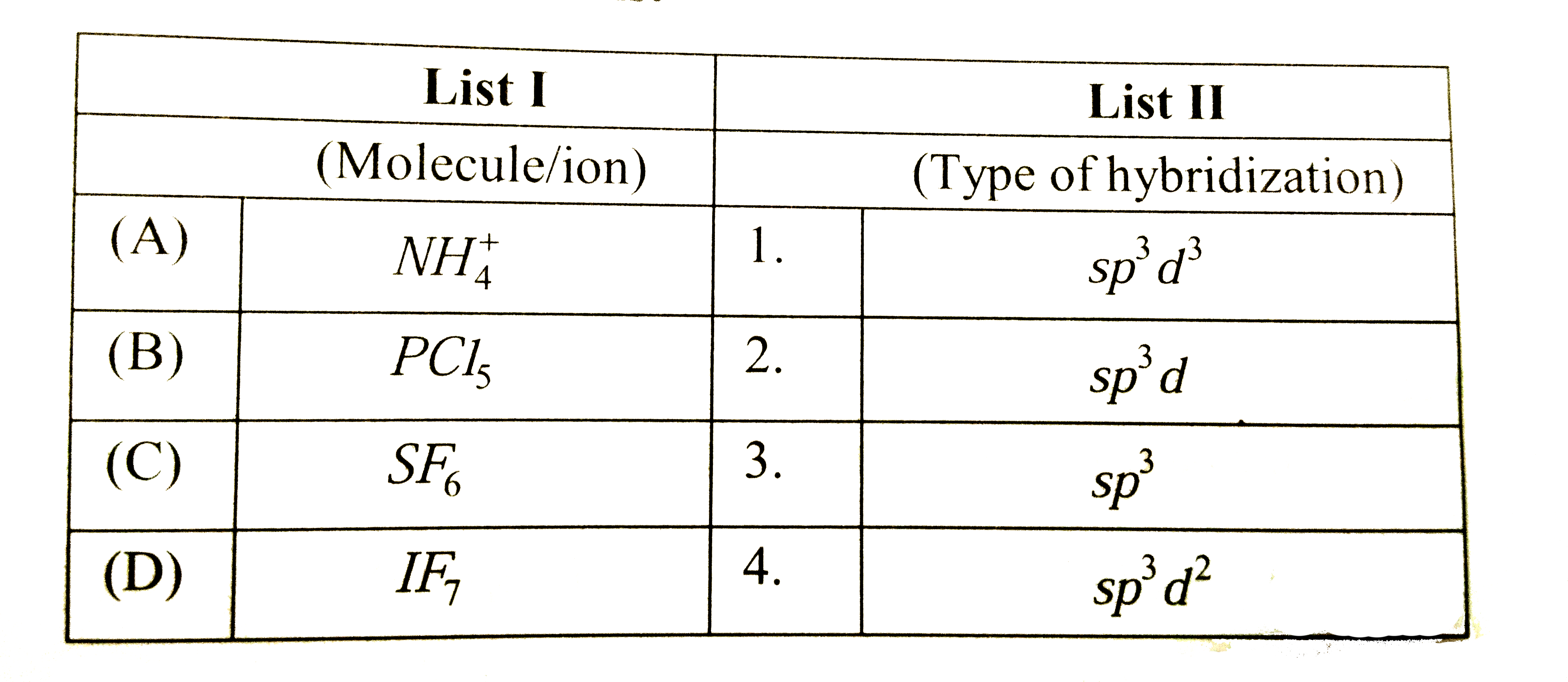
An aqeous solution of X is added slowly to an aqueous solution of Y as shown in List-I. The variation in conductivity of these reaction in List-II. Match List-I with List-II and select the correct answer using the code given below the lists:
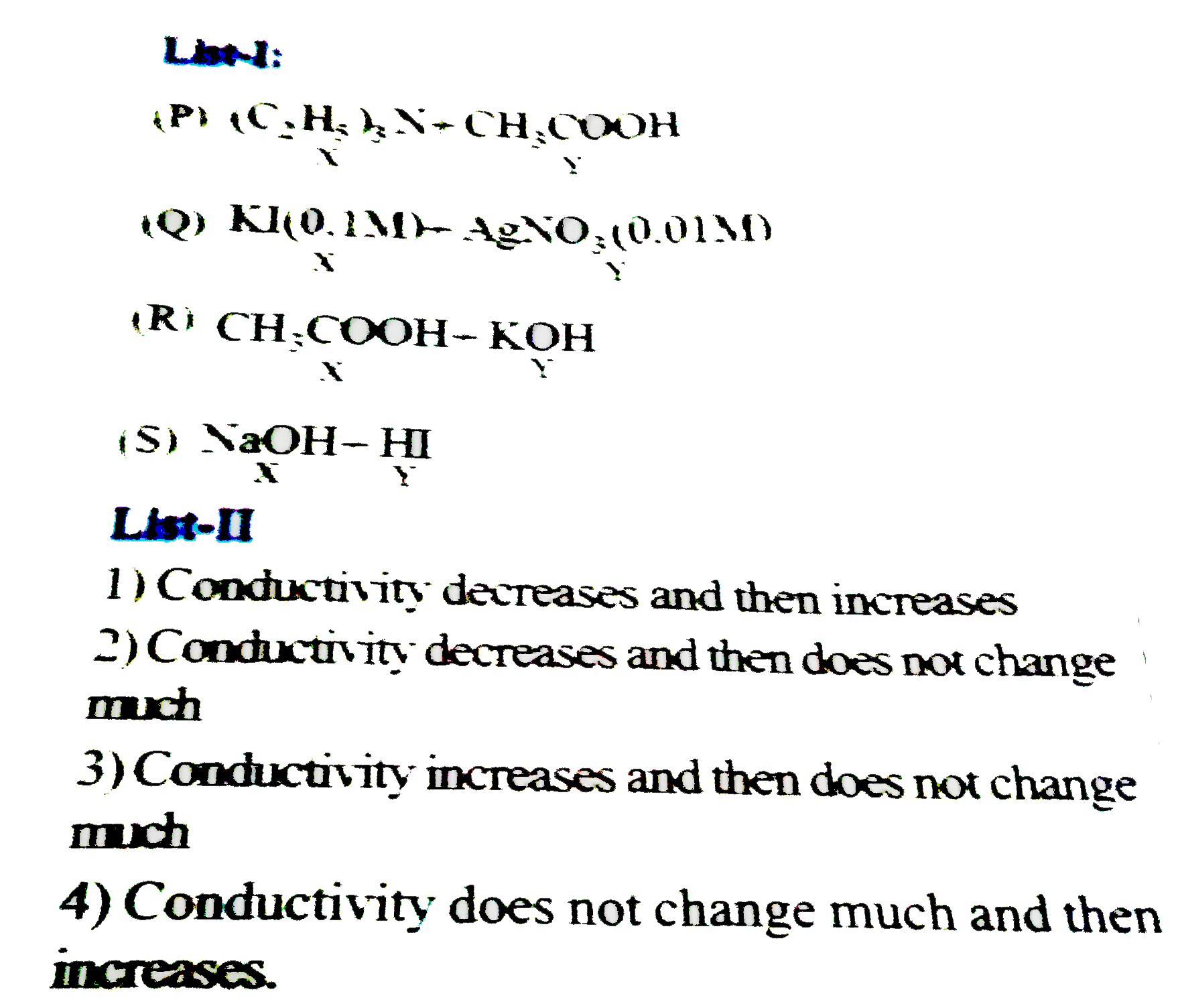
PQRS3421
PQRS4321
PQRS2341
PQRS1432
The correct Answer is:A
Step by step video & image solution for An aqeous solution of X is added slowly to an aqueous solution of Y as shown in List-I. The variation in conductivity of these reaction in List-II. Match List-I with List-II and select the correct answer using the code given below the lists: by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- JEE ADVANCEDBook:JEE ADVANCED PREVIOUS YEARChapter:JEE ADVANCEDExercise:SECTION- 1 (ONE OR MORE THAN ONE OPTIONS CORRET TYPE)
Explore 20 Videos
- JEE ADVANCEDBook:JEE ADVANCED PREVIOUS YEARChapter:JEE ADVANCEDExercise:PART-2 CHEMISTRY SECTION -1 (ONLY ONE OPTION CORRECT TYPE)
Explore 10 Videos
- JEE ADVANCEDBook:JEE ADVANCED PREVIOUS YEARChapter:JEE ADVANCEDExercise:PART II: CHEMISTRY (Section -2):(Paragraph Type)
Explore 8 Videos
- JEE (ADVANCED ) 2020Book:JEE ADVANCED PREVIOUS YEARChapter:JEE (ADVANCED ) 2020Exercise:SECTION -III
Explore 6 Videos
- JEE ADVANCED 2020Book:JEE ADVANCED PREVIOUS YEARChapter:JEE ADVANCED 2020Exercise:SECTION-3
Explore 6 Videos
Similar Questions
Knowledge Check
Match List-I with List -II and select correct correct answer using the code given below the lists.
 A
A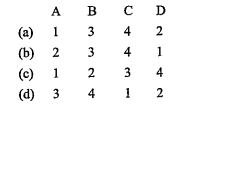 B
B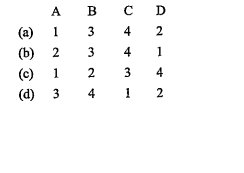 C
C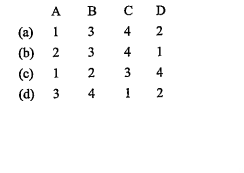 D
D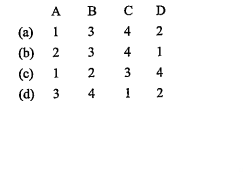
Match list I what list II and select the correct answer using the correct codes given below the lists:
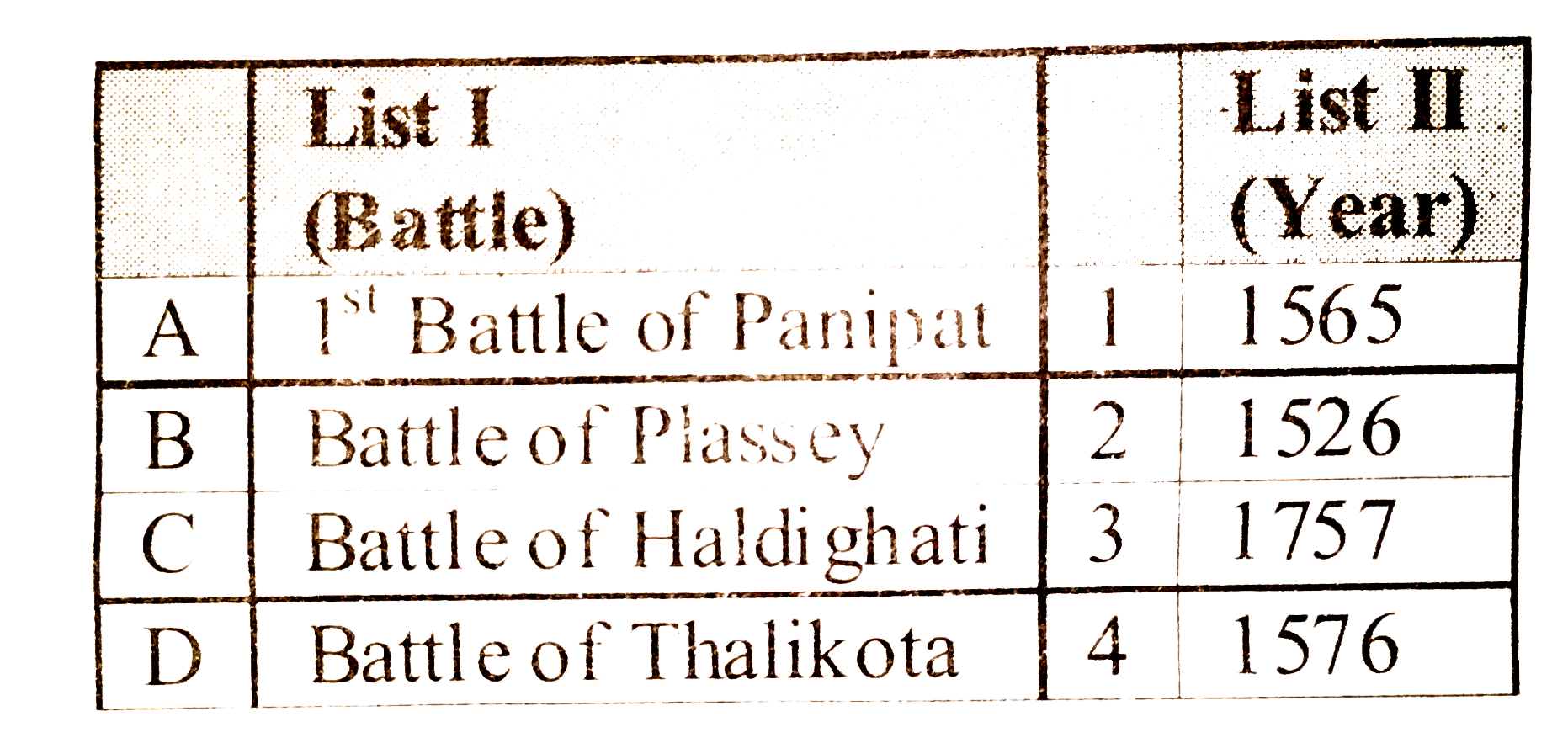 AA-2,B-3,C-4,D-1BA-2,B-4,C-3,D-1CA-1,B-4,C-3,D-2DA-1,B-3,C-4,D-2
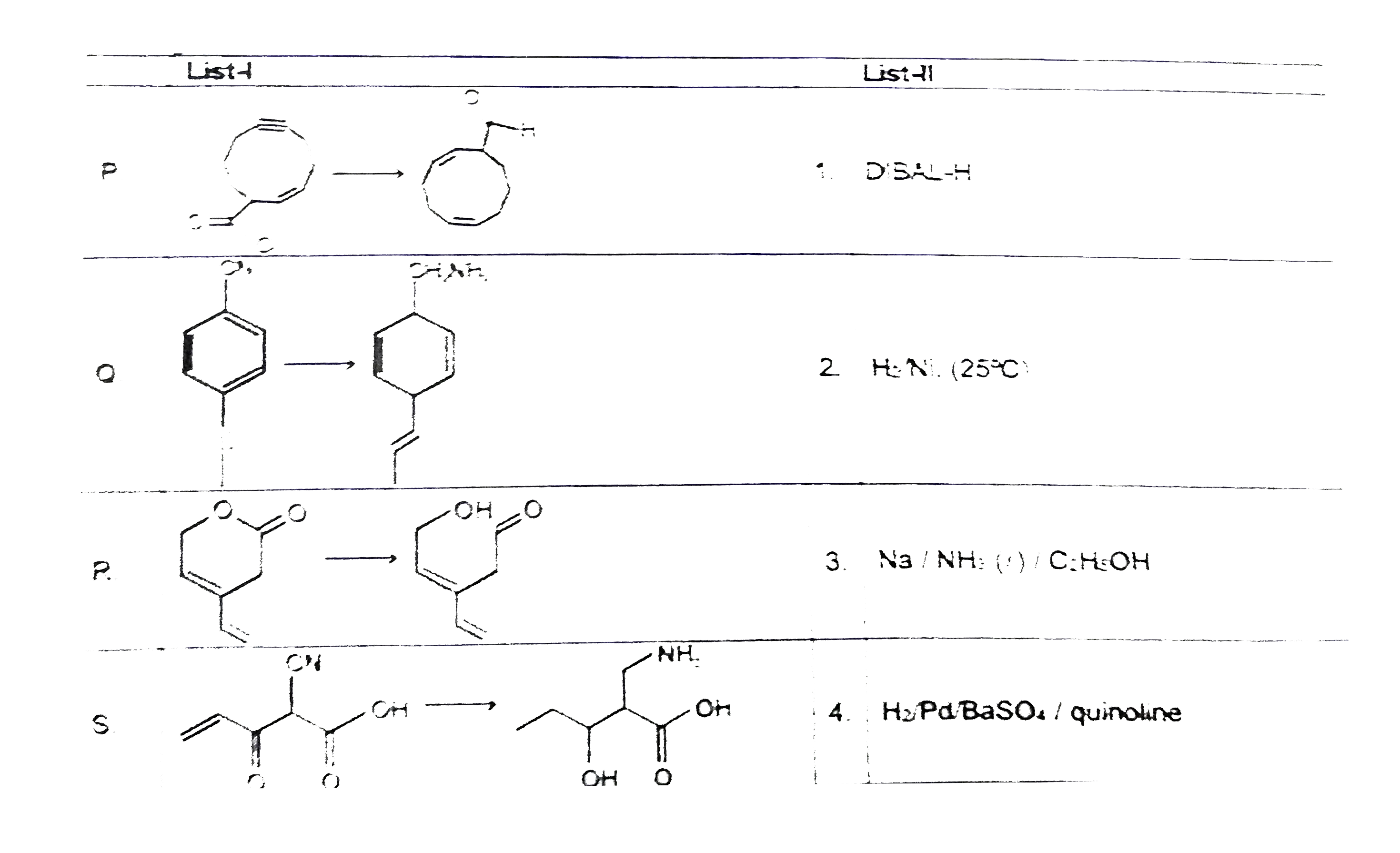
AA-2,B-3,C-4,D-1BA-2,B-4,C-3,D-1CA-1,B-4,C-3,D-2DA-1,B-3,C-4,D-2Match List-I (reaction) with List-II (name of the reaction) and select the correct answer using the codes given below the lists:
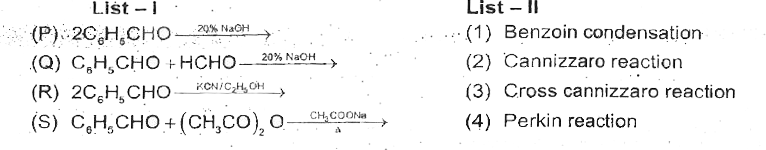 APQRS1324BPQRS2314CPQRS1342DPQRS2341
APQRS1324BPQRS2314CPQRS1342DPQRS2341
Match List I (Reaction) with List II (Type of reaction ) and select the correct answer using the code given below the lists :
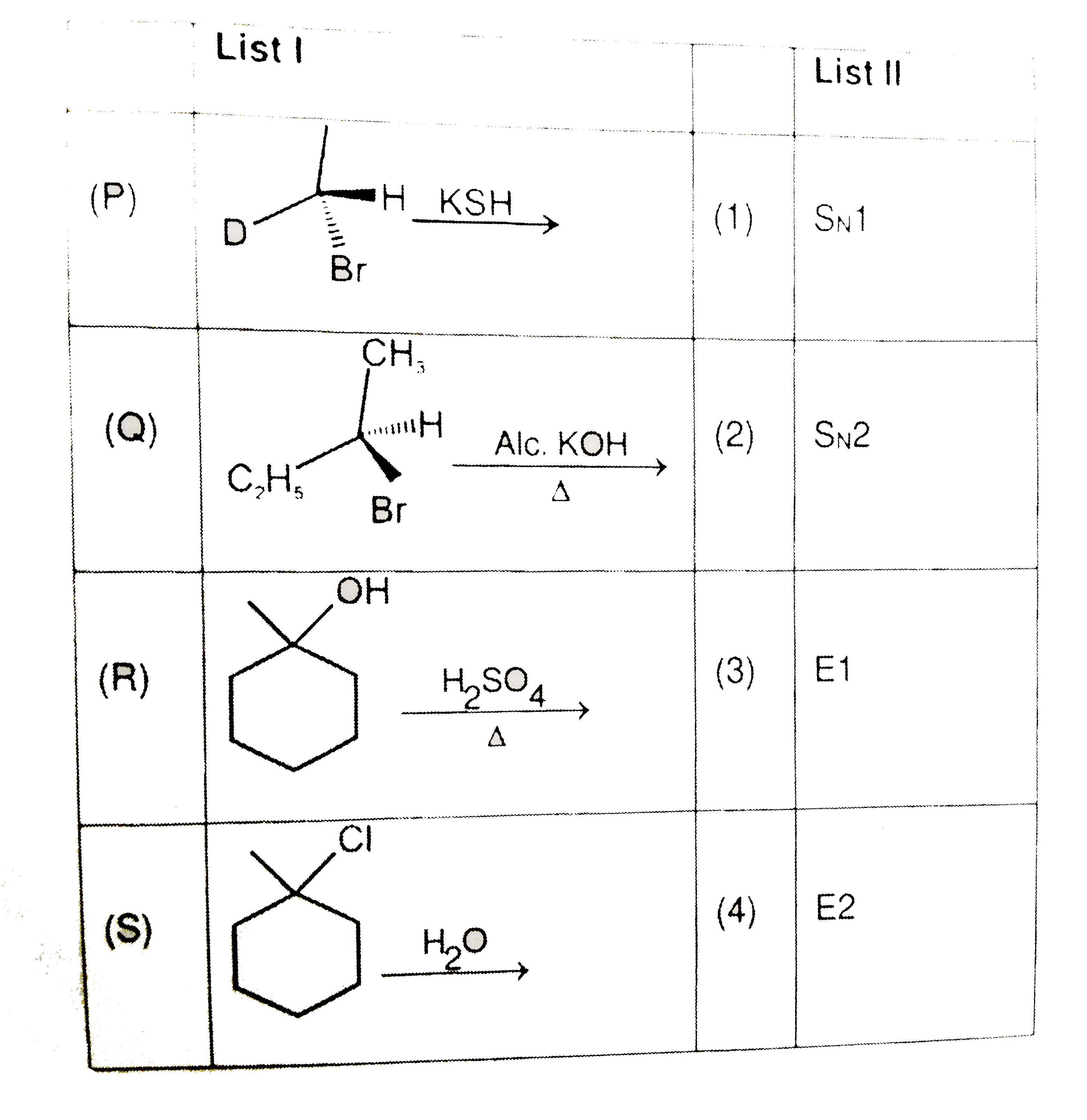
View SolutionMatch List I (Reaction ) with List II (Type of reaction ) and select the correct answer using the code given below the lists :
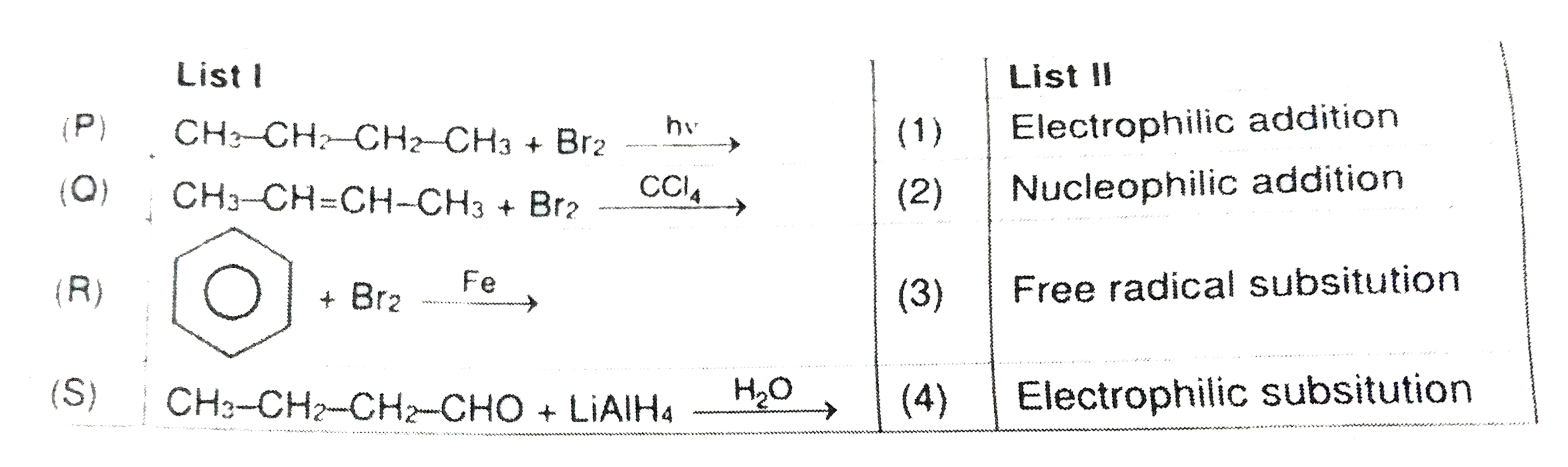
View SolutionMatch List I (Reaction) with List II (Type of reaction ) and select the correct answer using the code given below the lists :
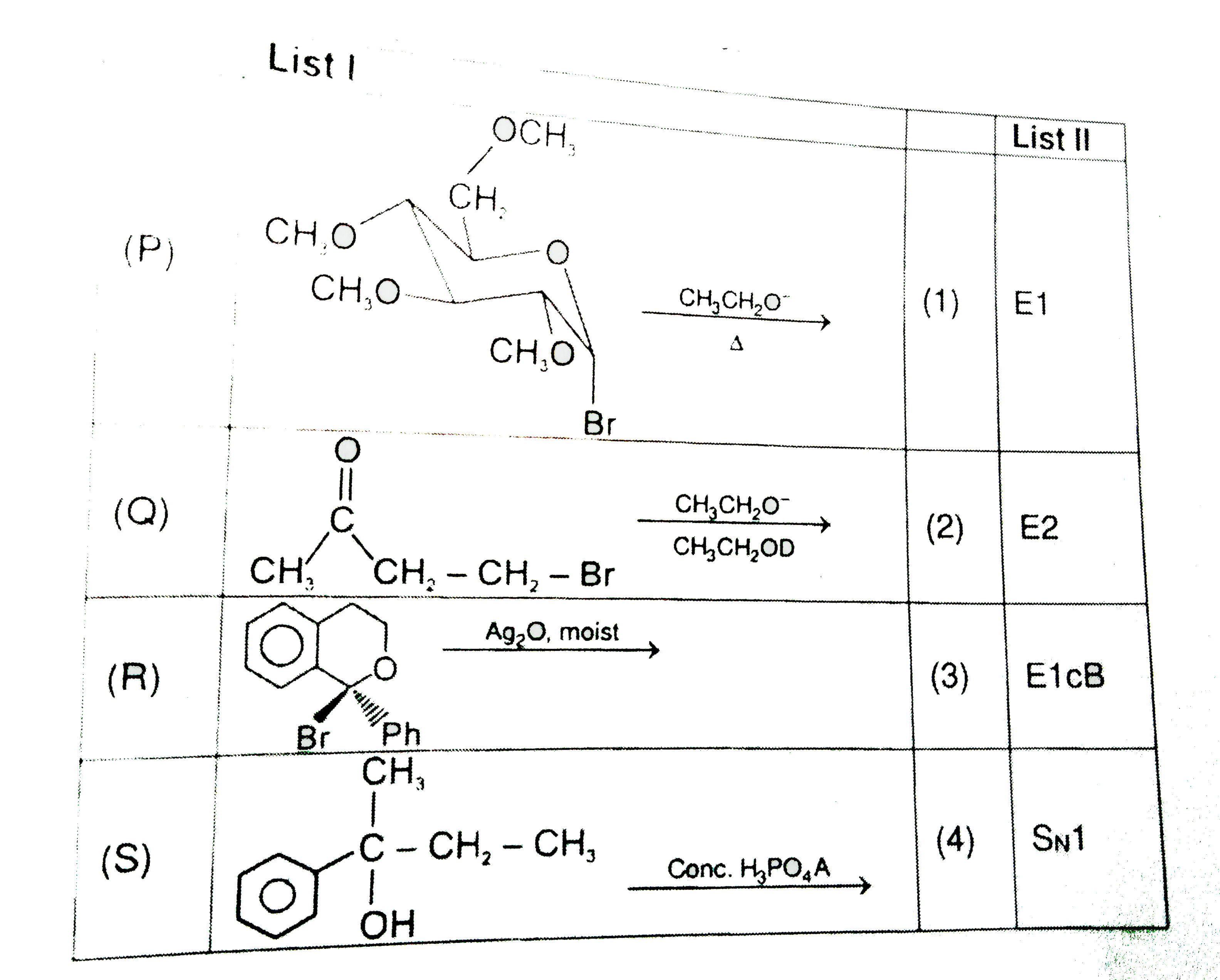
View Solution