The table shown below gives the bond dissociation emergies (Ediss) for single covalent bonds of carbon (C) atoms with element A,B,C and D. which element has the smallest atoms

A
B
C
D
The correct Answer is:D
Bond dissociation energy decreases with increases in size. So D is smallest
Topper's Solved these Questions
- CHEMICAL BONDING BOOK - ERRORLESS CHAPTER - CHEMICAL BONDING EXERCISE - Ordinary thinking (Coordinate or dative bonding )
15 Videos
- CHEMICAL BONDING BOOK - ERRORLESS CHAPTER - CHEMICAL BONDING EXERCISE - Ordinary thinking (Dipole moment )
34 Videos
- CHEMICAL BONDING BOOK - ERRORLESS CHAPTER - CHEMICAL BONDING EXERCISE - JEE Section (Jee (advanced ) 2018)
1 Video
- CARBOXYLIC ACID AND THEIR DERIVATIVESBOOK - ERRORLESS CHAPTER - CARBOXYLIC ACID AND THEIR DERIVATIVESEXERCISE - Jee Section (Matrix Match type Questions)
2 Videos
- CHEMICAL EQULIBRIUMBOOK - ERRORLESS CHAPTER - CHEMICAL EQULIBRIUMEXERCISE - JS JEE SECTION (ONLY ONE CHOICE ANSWER (Matrix)
1 Video
Similar Questions
The H-H bond dissociation enthalpy of H2 is _________, is the highest for a single bond between two atoms of any elements.
View SolutionThe atomic number of three elements A, B and C are given below :
ElementABCAtomic number5710
Answer the following:
(a) Write the group number of the three elements in the Modern Periodic Table.
(b) Mention the period of these elements in the Modern Periodic Table.
(c) Out of these three elements which one has the smallest atomic radius and why ?
View Solution
Knowledge Check
The table shown below gives the bond dissociation energies (Ediss) for single covalent bonds of carbon (C) atoms with element A, B, C and D. Which element has the smallest atoms?
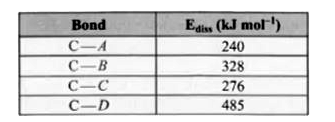 ACBDCADB
ACBDCADBThe table shown below gives the bond dissociation energies (Ediss) for single covalent bonds of carbon (C ) atom with element A,B,c and D. Which element out of these has the smallest atoms ?
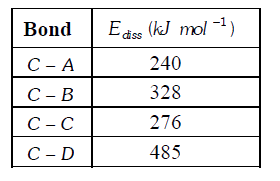 AABBCCDD
AABBCCDDThe table given below lists the bound dissociation enegrgy (Ediss) for single covelent bonds formed between C and atom A,B,D,E.

What of the atoms has smallest size?ADBECADB
The table given below lists the bond dissociation energy (Ediss) for single covalent bonds formed between C and atoms A, B, D, E.
BondEdiss(kcal mol−1)C−A 240C−B 382C−D 276C−E 486
Which of the atoms has smallest size ?
View SolutionWhich mentioned bond has highest C−H bond dissociation energy.
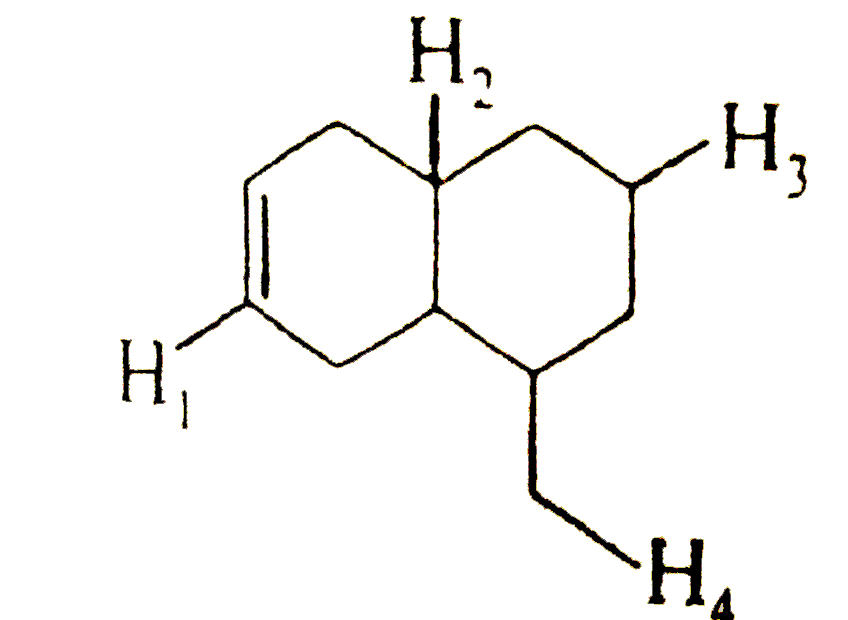
View SolutionWhich element-element bond has the highest bond dissociation energy ?
View SolutionWhich of the following C-H bond has the lowest bond dissociation energy
View SolutionWhich is the correct order of bond energy of single, double and triple bonds between carbon atoms ?
View Solution

