Which molecules does not show zero dipole moment
A
BF3
B
NH3
C
CCl4
D
CH4
Text Solution
The correct Answer is:B
Ammonium have some dipole moment
|
Topper's Solved these Questions
- CHEMICAL BONDING Book:ERRORLESS Chapter:CHEMICAL BONDING Exercise:Ordinary thinking (Polarisation and Fajans rule )
Explore 29 Videos
- CHEMICAL BONDING Book:ERRORLESS Chapter:CHEMICAL BONDING Exercise:Ordinary thinking (Hybridisation )
Explore 133 Videos
- CHEMICAL BONDING Book:ERRORLESS Chapter:CHEMICAL BONDING Exercise:Ordinary thinking (Coordinate or dative bonding )
Explore 15 Videos
- CARBOXYLIC ACID AND THEIR DERIVATIVESBook:ERRORLESS Chapter:CARBOXYLIC ACID AND THEIR DERIVATIVESExercise:Jee Section (Matrix Match type Questions)
Explore 2 Videos
- CHEMICAL EQULIBRIUMBook:ERRORLESS Chapter:CHEMICAL EQULIBRIUMExercise:JS JEE SECTION (ONLY ONE CHOICE ANSWER (Matrix)
Explore 1 Video
Similar Questions
Knowledge Check
Which of the following molecules does not have net dipole moment?
ACH3−BrBCH2Cl2CHCOOHD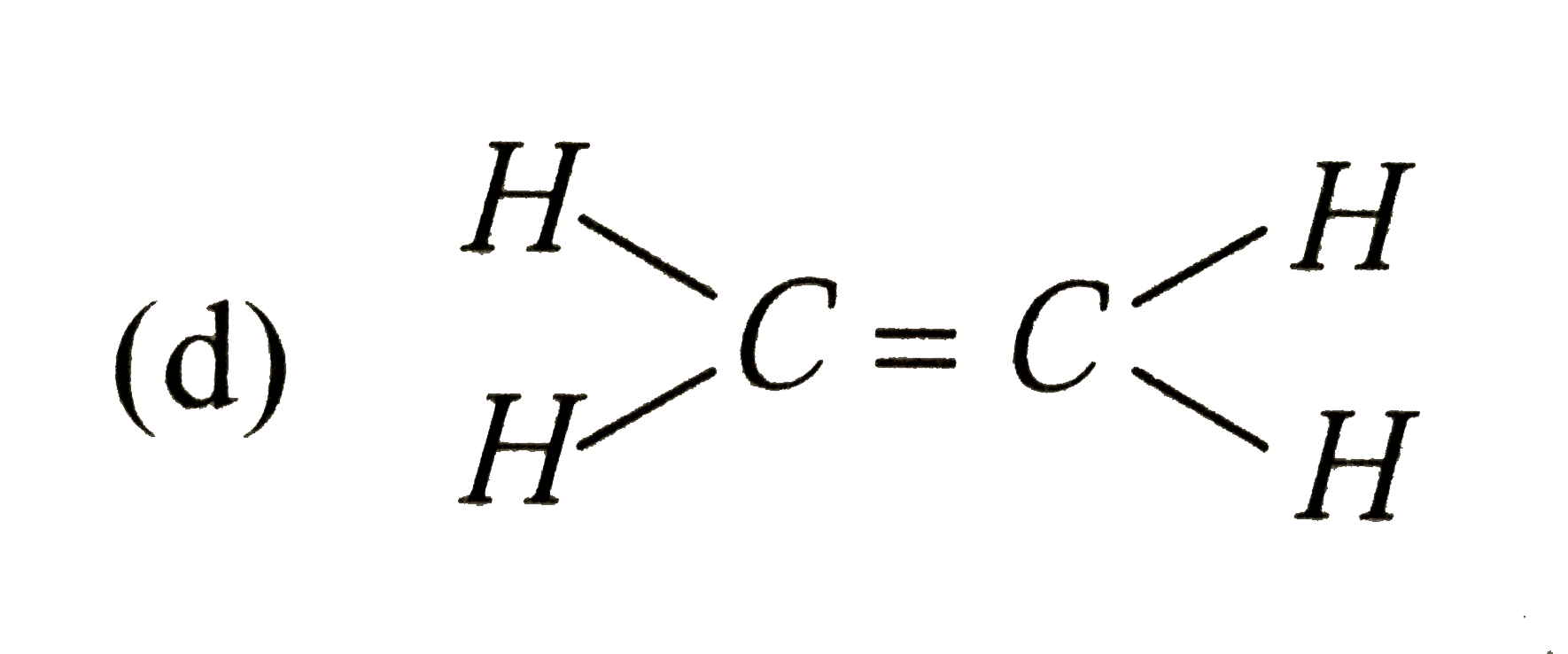
Which of the following molecules does not have net dipole moment?
ACH3−BrBCH2CI2C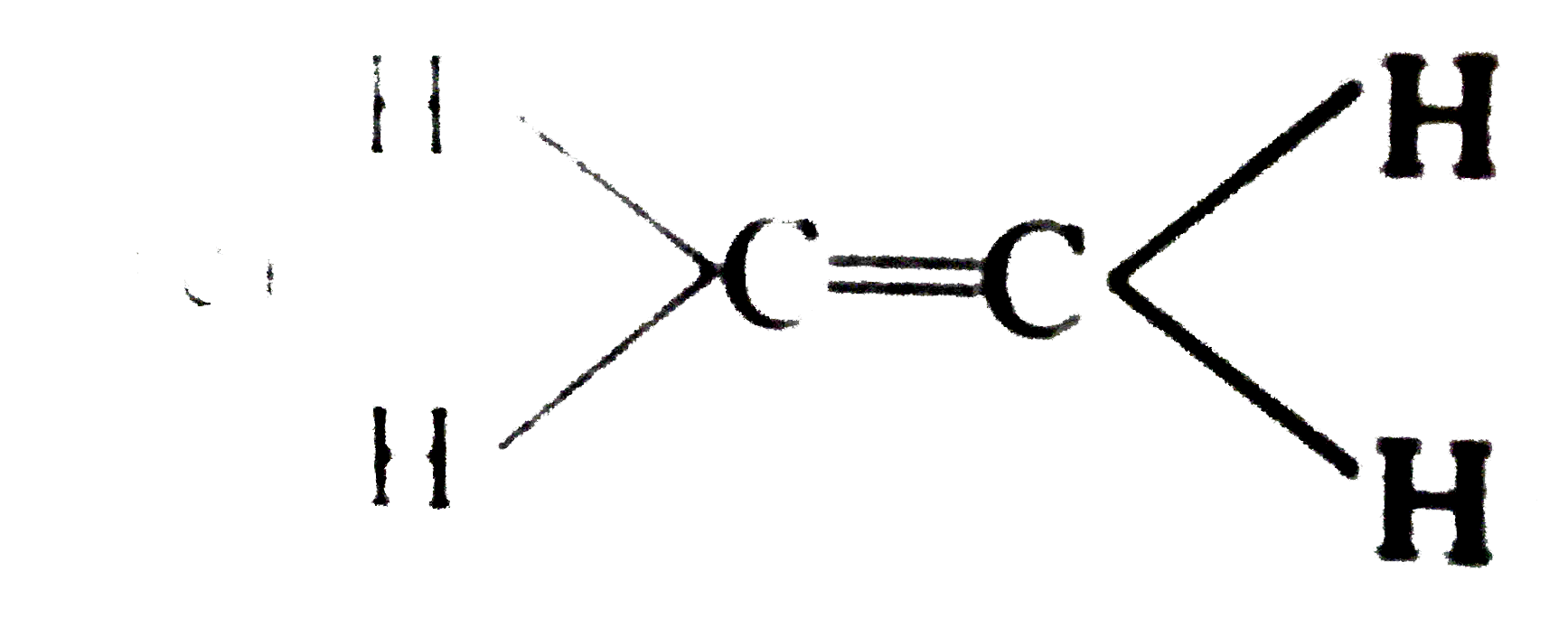 DHCOOH
DHCOOHWhich has zero dipole moment
ACis-2-buteneBTrans-2-buteneC1-buteneD2-methyl-1-propene

