The optically active co-ordination complex ion among the following is
A
trans [Co(en)2Cl2]+
B
cis [Co(en)(NH3)2Cl2]+
C
[Co(NH3)6]3+
D
[Fe(CN)6]3−
Video Solution
Text Solution
The correct Answer is:B
Octahedral complexes of the type [M(AA)2b2]n± and [M(AA)a2b2]n± exhibit geometrical isomerism.
Option(a) , i.e., trans - [Co(en)2Cl2]+ which belongs to the 1st category is optically inactive owing to presence of an element of symmetry . However (b), cis- [Co(en)(NH3)2Cl2]+ exists is optically active forms.
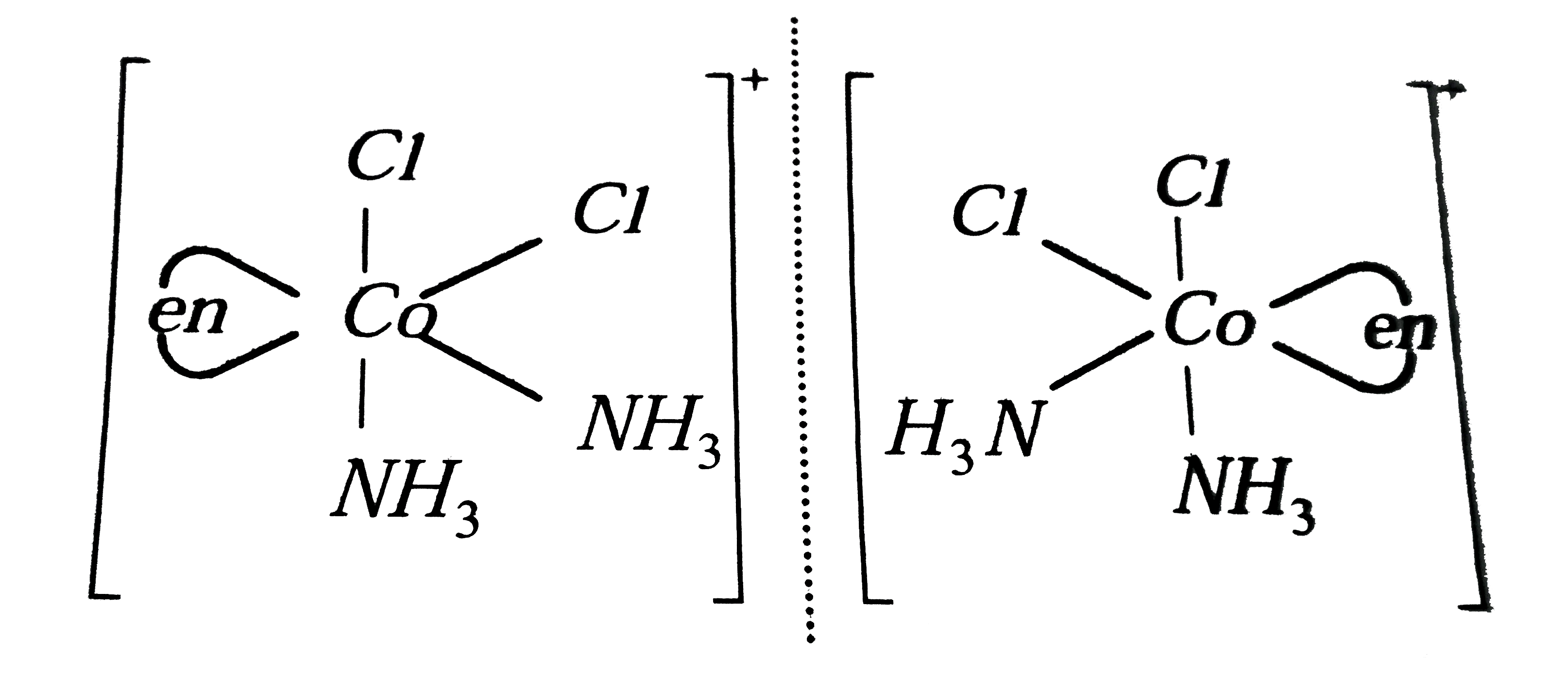
Options (c ) and (d) are optically inactive as all the ligands are same.
|
Similar Questions
Knowledge Check
The optically active complex are
A[As(CH3)(Cl)(Br)(I)]+B[Pt(NH3)(Py)(Cl)(Br)]C[Pt(gly)2]D[Cr(en)3]Cl3Which is the most stable & optically active conformer among the following ?
A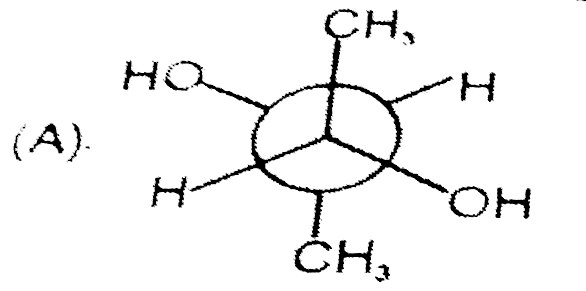 B
B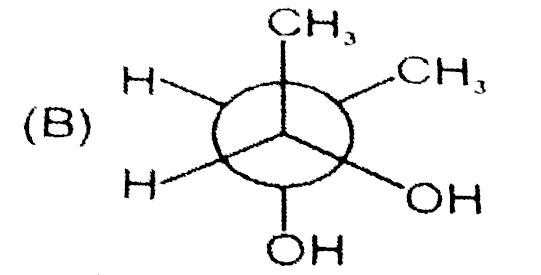 C
C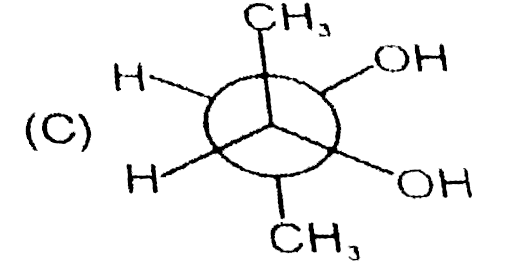 D
D
Select the optically active compound among the following :
A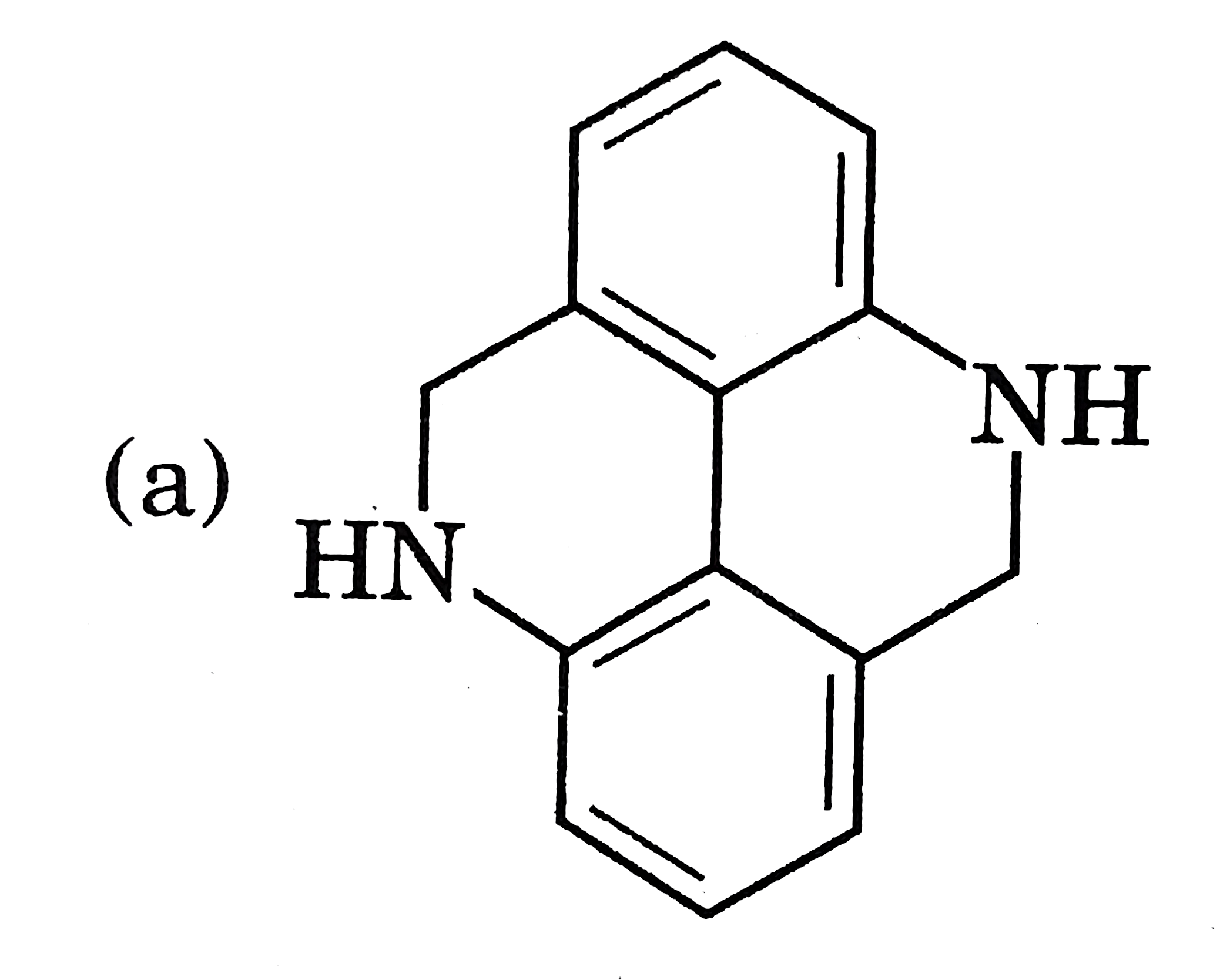 B
B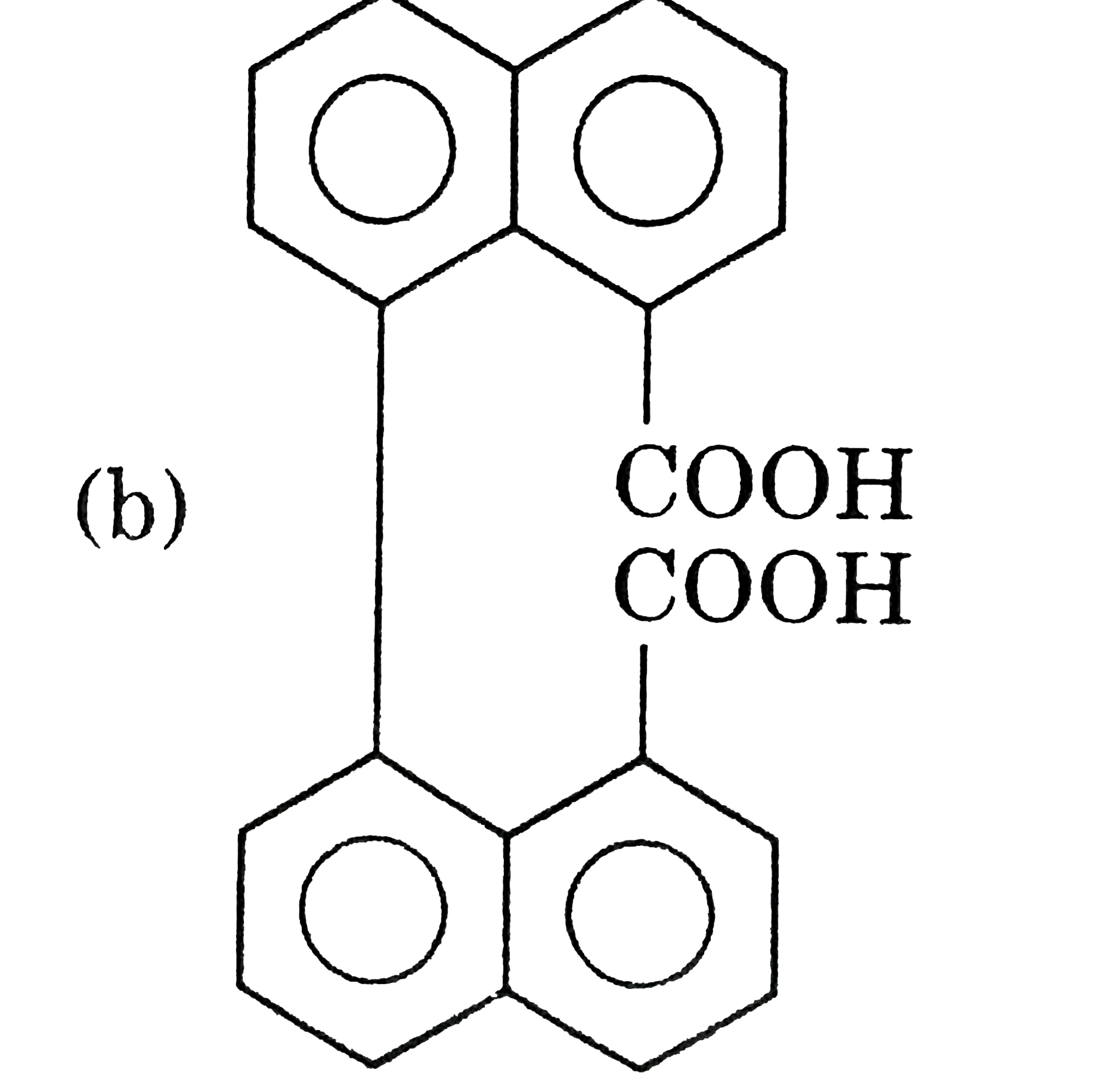 C
C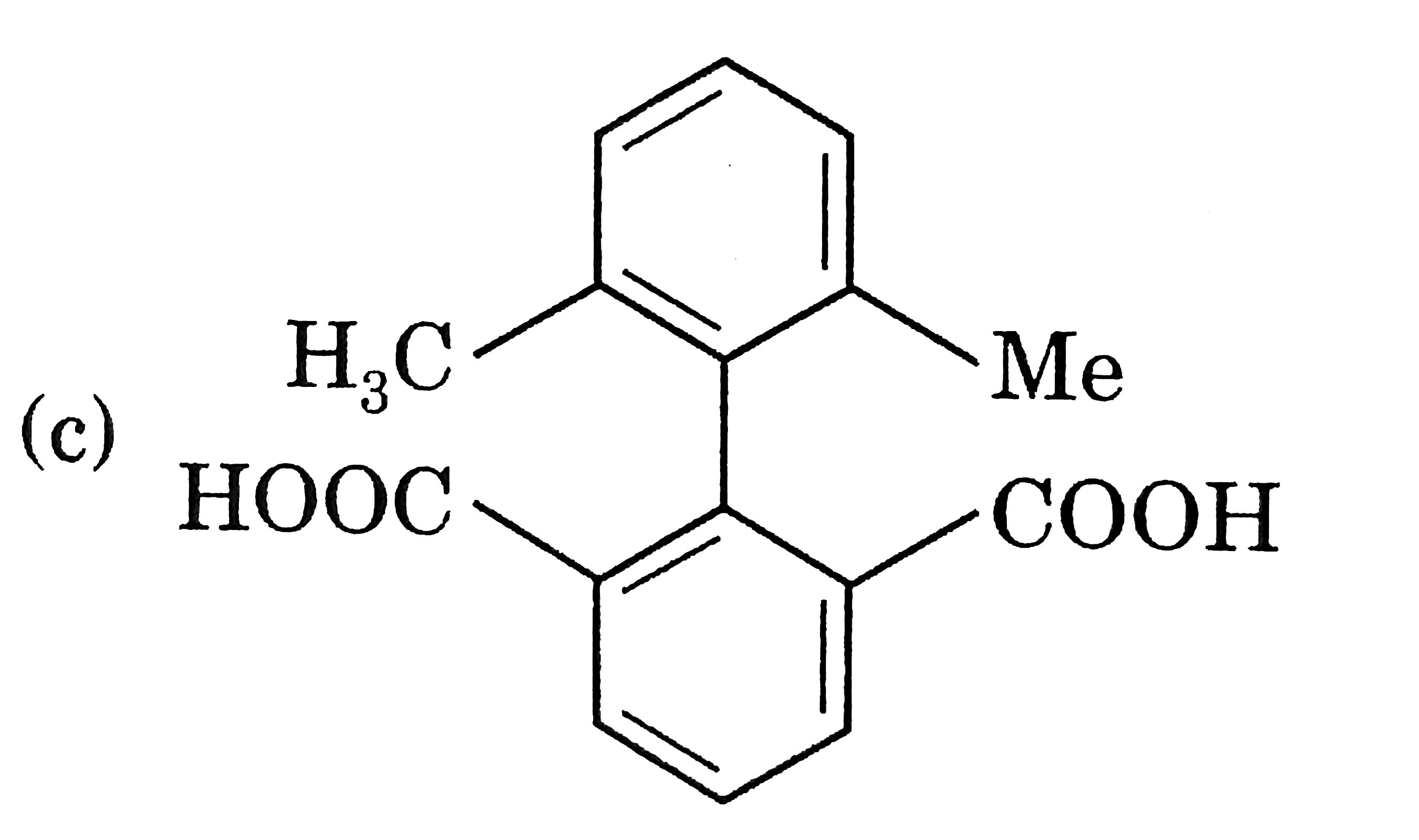 D
D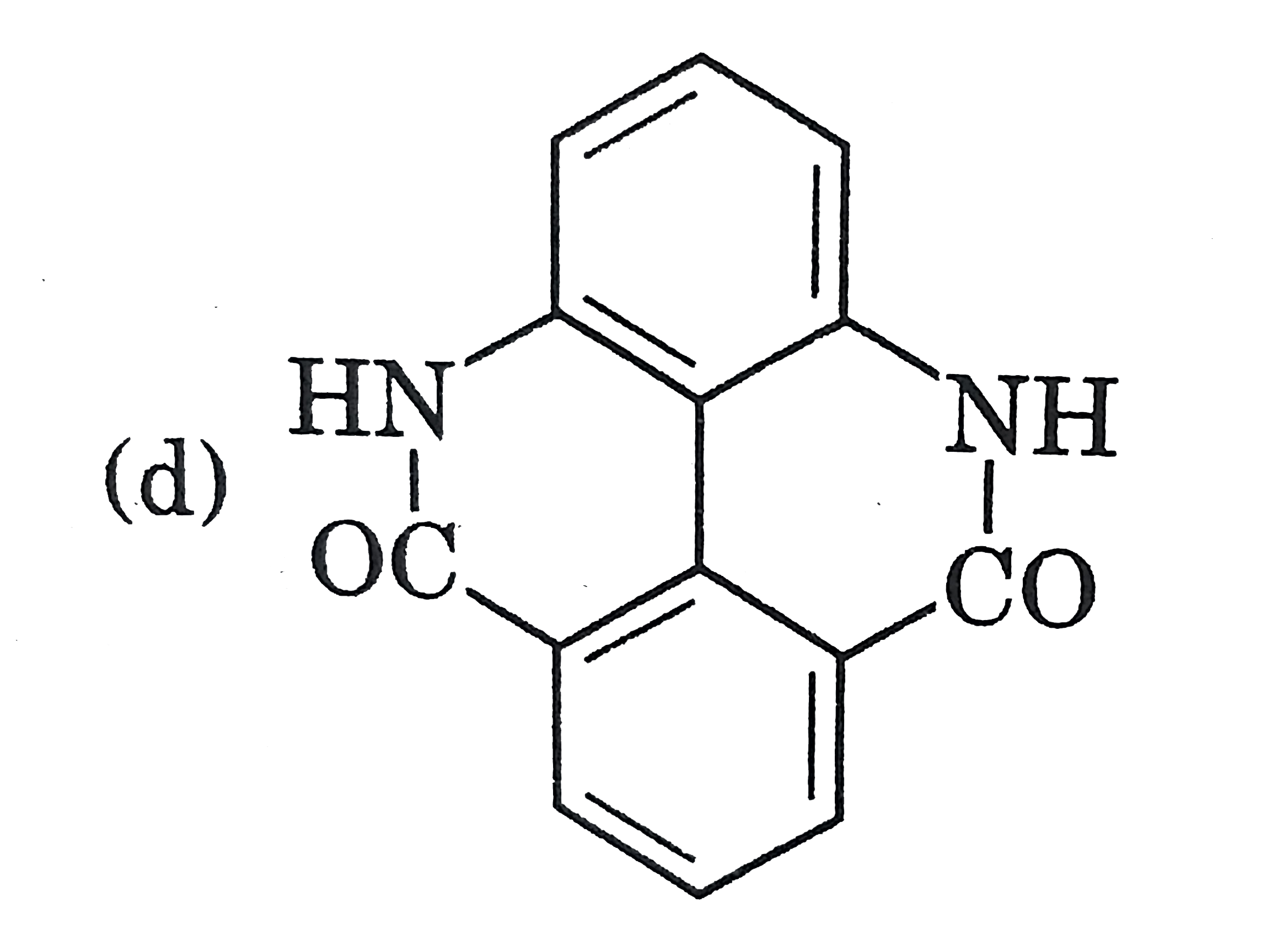
The optically active species among the following is:
View SolutionThe optically active species among the following is:
View SolutionEthers form co-ordination complexes with
View SolutionOptically active molecules among the following are
View SolutionThe complex ion which has the highest magnetic moment among the following is
View Solution
