Predict the number of unpaired electrons in the square planar [Pt(CN)4]2− ion.
A
Zero
B
1
C
4
D
6
Video Solution
Text Solution
The correct Answer is:A
Outer electronic configuration of Pt is 5d96s1 . Outer electronic configuration of Pt2+ is 5d8. As CN− is strong field ligand, so pairing will take place.
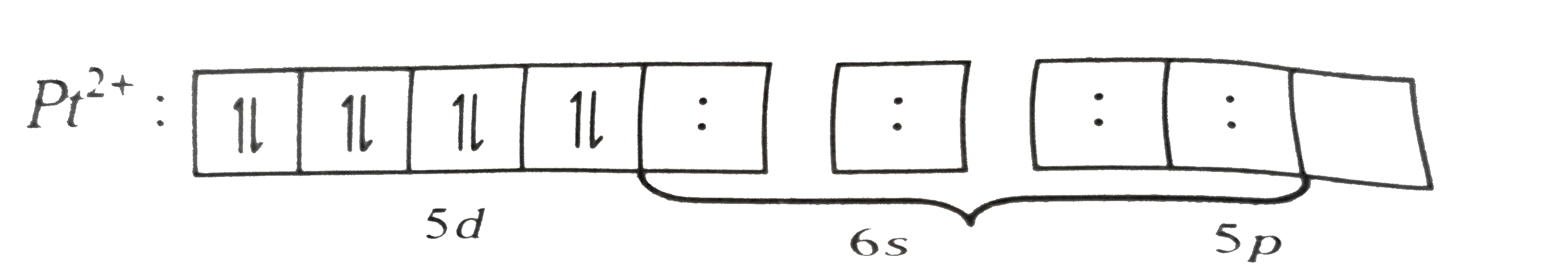
|
Similar Questions
Knowledge Check
The number of unpaired electrons in the square planar [Pt(CN)4]2− ion is
A2B1C0D3What is (are) number (s) of unpaired electron(s) in the square planar [Pt(CN)4]2− ion ?
AZeroB1C4D6What is (are) number (s) of unpaired electron(s) in the square planar [Pt(CN)4]2− ion ?
A0B1C4D6
Predict the number of unpaired electrons in a square planar d8 ion.
View SolutionNumber of unpaired electrons in N2+ is/are
View SolutionThe number of unpaired electrons in Zn2+ is
View SolutionThe number of unpaired electron in Ni2+ is
View SolutionNumber of unpaired electrons in Mn4+ is
View Solution

