Which of the following compound is produced when CH2=CH−(CH2)2COOH reacts with HBr in presence of peroxides
CH3CH(CH2)5COOH
BrCH2CH2(CH2)2COOH
CH3CH2CH2(CH2)5COOH
CH3CH2BrCH2CH2COOH
The correct Answer is:B
To solve the problem of what compound is produced when CH₂=CH-(CH₂)₂COOH reacts with HBr in the presence of peroxides, we can follow these steps:
1. Identify the Reactants: The reactant is CH₂=CH-(CH₂)₂COOH, which is an alkene (specifically, an unsaturated fatty acid).
2. Understand the Reaction Conditions: The reaction occurs in the presence of HBr and peroxides. The presence of peroxides indicates that we will have a radical mechanism, specifically anti-Markovnikov addition.
3. Radical Formation: When HBr is added in the presence of peroxides, the peroxide decomposes to form bromine radicals (Br•). This is crucial because it leads to the anti-Markovnikov addition of HBr.
4. Addition of Bromine Radical: The Br• radical will add to the less substituted carbon of the double bond (the terminal carbon in this case). This is because the radical mechanism favors the formation of the more stable radical.
5. Formation of the Radical: After the Br• adds to the terminal carbon (CH₂), a radical is formed on the adjacent carbon (the one that was originally part of the double bond). This results in the structure:
- CH₂Br-CH•-CH₂-COOH
6. Final Product Formation: The radical can then react with HBr to form the final product. The hydrogen from HBr will add to the carbon with the radical, resulting in:
- CH₂Br-CH₂-CH₂-COOH
7. Conclusion: The final product after the reaction is CH₂Br-(CH₂)₂COOH, which corresponds to option 2.
Final Answer:
The compound produced is CH₂Br-(CH₂)₂COOH.
- CH₂Br-CH•-CH₂-COOH
- CH₂Br-CH₂-CH₂-COOH
The compound produced is CH₂Br-(CH₂)₂COOH.
Similar Questions
Knowledge Check
CH2=C(CH2)2COOH and HBr reacts on presence of peroxide to give
ABrCH2CH2(CH2)2COOHBCH3CH(CH2)2COOHCCH3CHCH2(CH2)2COOHDCH3CHBr(CH2)2COOHExcess of CH3COOH is reacted with CH≡CH in presence of Hg2+, the product is -
ACH3(OOCCH3)|CH2(OOCH3)BCH3|CH2−(OOC−CH3)CCH3|CH(OOC−CH3)2DNone of theseBrCH2−CH2CH2Br reacts with Na in the presence of ether at 100∘C to produce
ABrCH2−CH=CH2BCH2=C=CH2C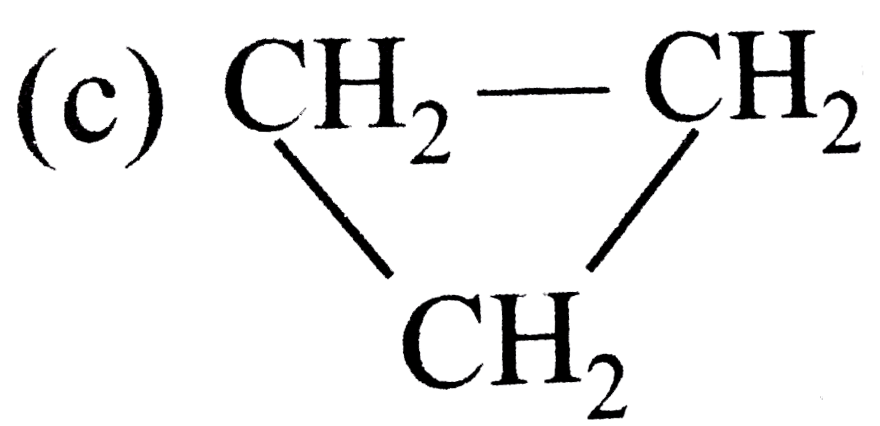 DAll of these
DAll of these
Excess of CH3COOH is reacted with CH≡CH in presence of Hg2+, the product is -
View SolutionWhich of the following compounds can liberate methane when treated with excess of methyl magnesium iodide in dry ether.
(a) CH3−CH2−CH2OH
(b) CH3−CH2−C≡CH
(c) CH3−CH2−COOH
(d) CH3−CH2−CHO
View SolutionThe treatment of CH3CH2COOH with chlorine in the presence of phosphorus gives:
View SolutionWhen CH2=CH−Br is reacted with HBr then the product formed is A and when CH2=CH−COOH is treated with HBr then the product formed is C. Hence here
View SolutionThe reaction of HBr with CH3C(CH3)=CH2 in the presence of peroxide will give
View Solution

