Which of the following compound is produced when CH2=CH−(CH2)2COOH reacts with HBr in presence of peroxides
A
CH3CH(CH2)5COOH
B
BrCH2CH2(CH2)5COOH
C
CH3CH2CH2(CH2)5COOH
D
CH3CH2BrCH2CH2COOH
Text Solution
The correct Answer is:B
|
Similar Questions
Knowledge Check
Which of the following compound is produced when CH2=CH−(CH2)2COOH reacts with HBr in presence of peroxides
ACH3CH(CH2)5COOHBBrCH2CH2(CH2)2COOHCCH3CH2CH2(CH2)5COOHDCH3CH2BrCH2CH2COOHCH2=C(CH2)2COOH and HBr reacts on presence of peroxide to give
ABrCH2CH2(CH2)2COOHBCH3CH(CH2)2COOHCCH3CHCH2(CH2)2COOHDCH3CHBr(CH2)2COOHBrCH2−CH2CH2Br reacts with Na in the presence of ether at 100∘C to produce
ABrCH2−CH=CH2BCH2=C=CH2C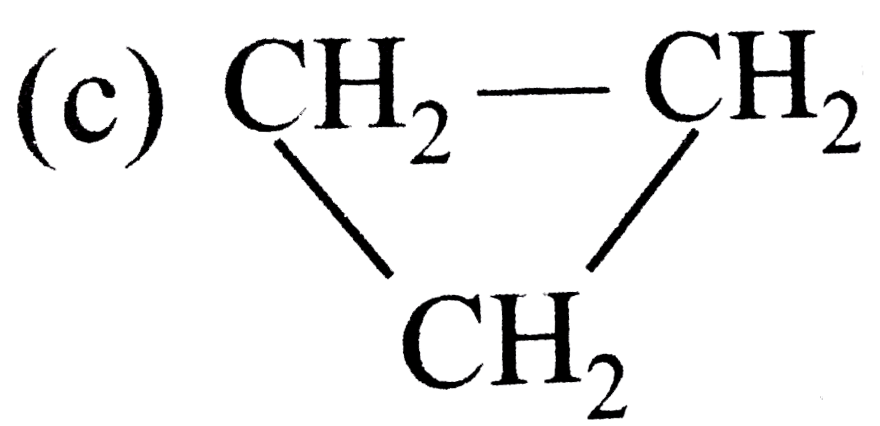 DAll of these
DAll of these
Write IUPAC name of the following compound :
CH3CH2CH(CHO)CH2COOH
View SolutionWrite IUPAC names of the following compounds :
CH3CH2COOH
View SolutionExcess of CH3COOH is reacted with CH≡CH in presence of Hg2+, the product is -
View SolutionCH2=CH−(CH2)5COOHPeroxide−−−−→HBrZ where Z is _________.
View SolutionCH2=CH−(CH2)5COOHPeroxide−−−−→HBrZ where Z is _________.
View Solution

