Which one of the following is not aromatic ?
A
Cyclopentadienyl cation
B
Cyclooctatetraene
C
Cycloheptatriene
D
Cycloheptatrienyl cation
Video Solution
Text Solution
The correct Answer is:D
To be aromatic
(i)Molecule should have (4n+2)π electrons in conjugation
(ii)Molecular should be planar Cycloheptatriene (4n+2)π electrons but not in conjugation
|
Topper's Solved these Questions
- HYDROCARBONBook:ERRORLESS Chapter:HYDROCARBONExercise:CRITICAL THINKING (Objective questions)
Explore 24 Videos
- HYDROCARBONBook:ERRORLESS Chapter:HYDROCARBONExercise:JEE SECTION (Only one choice correct answer)
Explore 93 Videos
- HYDROCARBONBook:ERRORLESS Chapter:HYDROCARBONExercise:ORDINARY THINKING (Objective question) (ALKYNE)
Explore 120 Videos
- HALOGEN CONTAINING COMPOUNDBook:ERRORLESS Chapter:HALOGEN CONTAINING COMPOUNDExercise:Critical Thinking (Jee (Advanced) 2018)
Explore 1 Video
- IONIC EQUILIBRIUMBook:ERRORLESS Chapter:IONIC EQUILIBRIUMExercise:JS Jee Section (JEE Advanced (Numeric answer type question))
Explore 1 Video
Similar Questions
Knowledge Check
Which one of the following is an aromatic compound ?
ACyclopentadienyl cationBCycloheptatrienyl cationCCycloheptatrienyl anionDCycloheptatrieneWhich one of the following is an aromatic compound ?
A B
B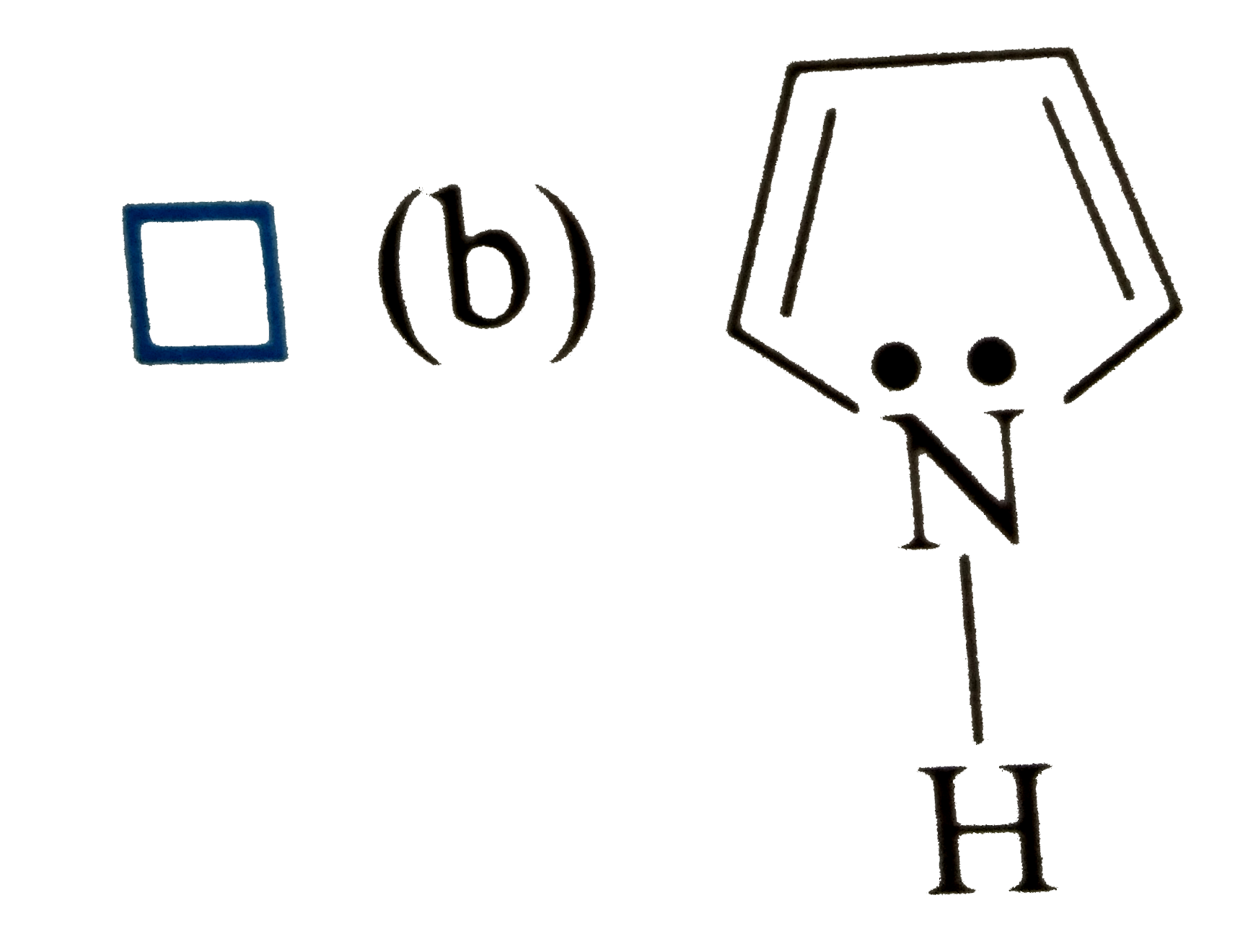 C
C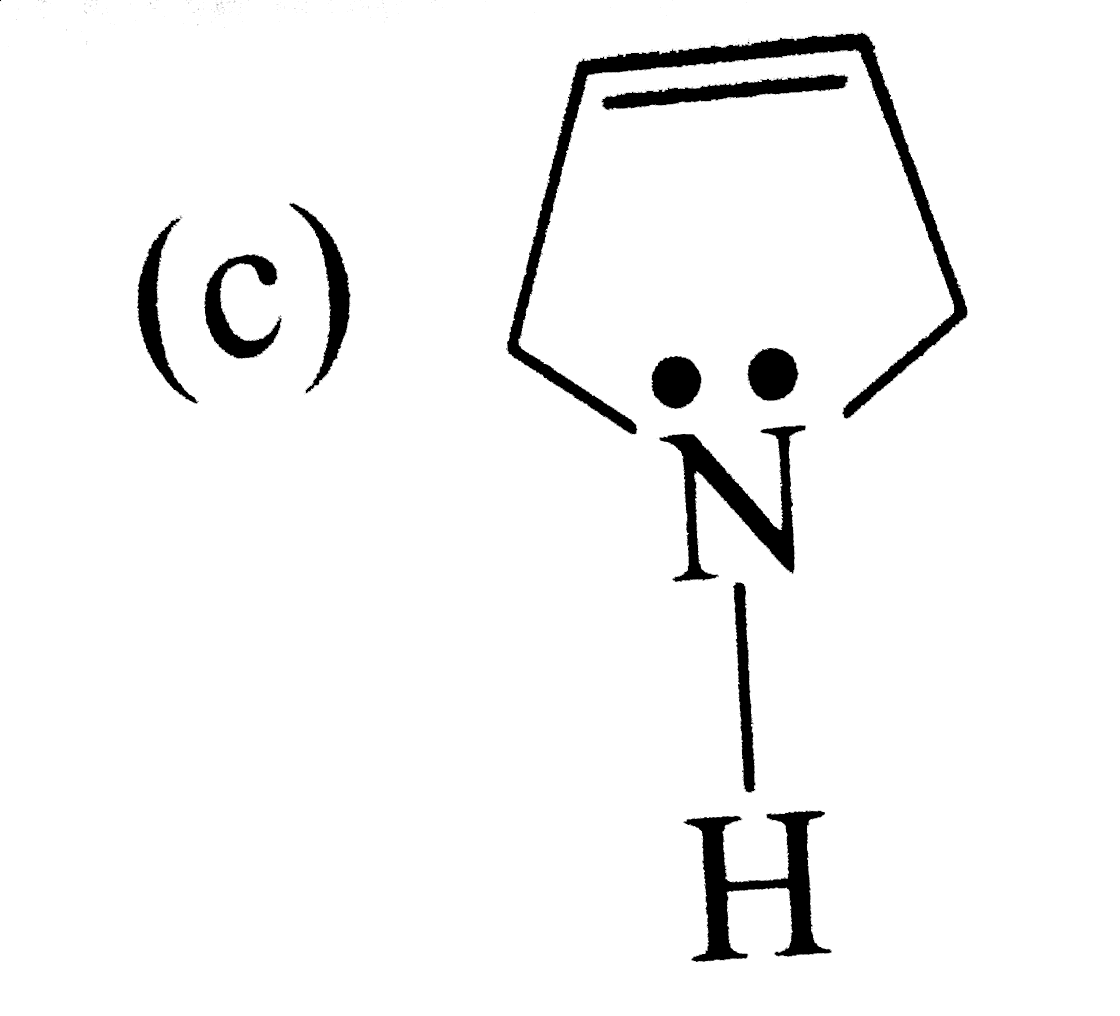 D
D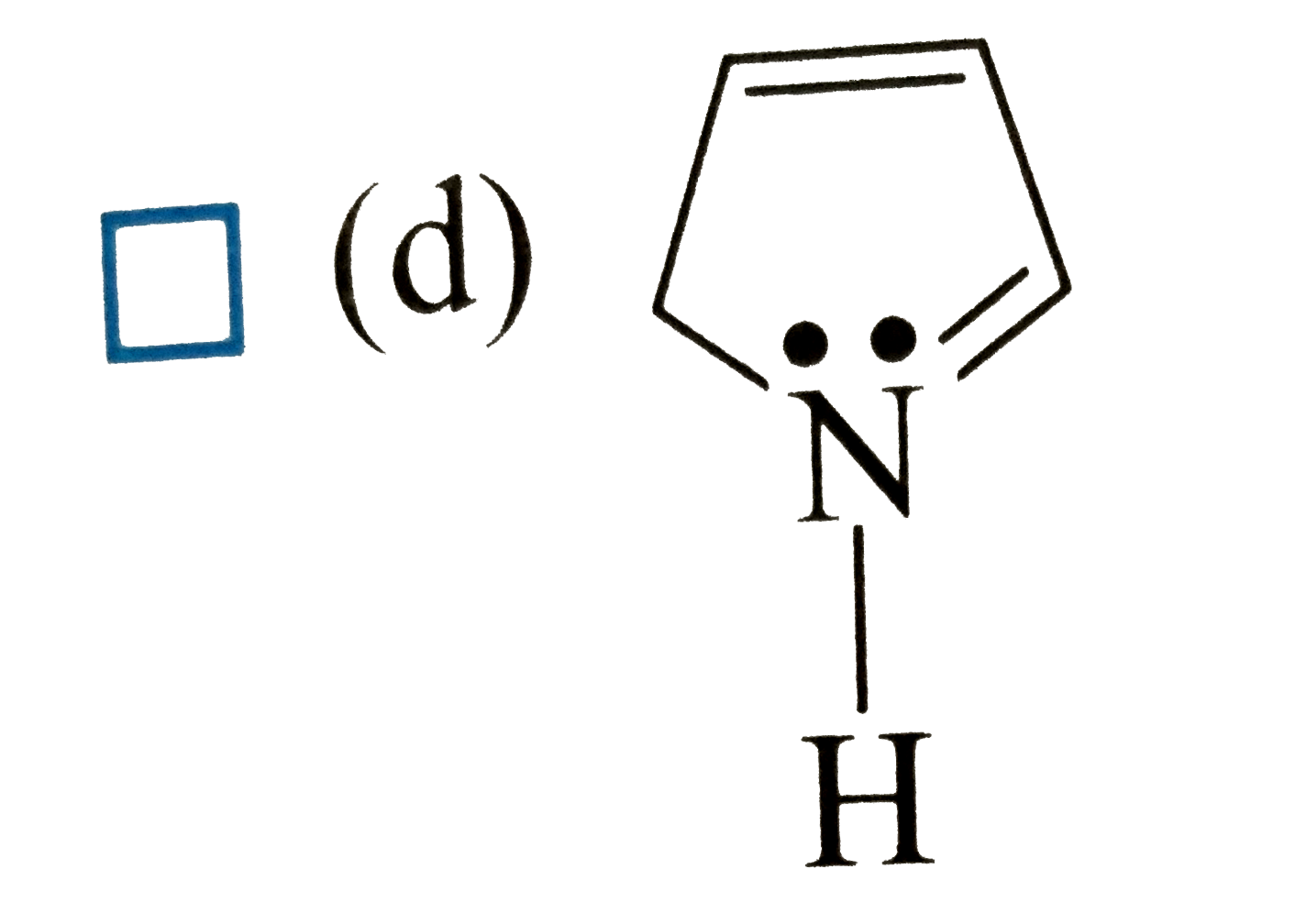
Which one of the following is an aromatic compound?
ACyclopentadienyl cationBCycloheptatrienyl cationCCycloheptatrienyl anionDCycloheptatriene
