Which of the following compounds is square planar and does have any unpaired electron?
A
Ni(CO)4
B
[Ni(H2O)6]2+
C
[NiCl4]2−
D
[Ni(CN)4]2−
Text Solution
[Ni(CN)4]2−
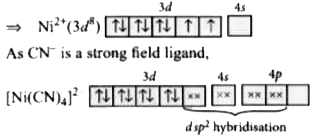
Therefore, hybridisation is dsp2, structure is square plnar and it is diamagnetic.
|
Topper's Solved these Questions
- COORDINATION COMPOUNDSBook:MTG-WBJEEChapter:COORDINATION COMPOUNDSExercise:WBJEE/WORKOUT (Category 2: Single Option Correct Type)
Explore 13 Videos
- COORDINATION COMPOUNDSBook:MTG-WBJEEChapter:COORDINATION COMPOUNDSExercise:WBJEE/WORKOUT (Category 3: One or More than one Option Correct Type)
Explore 7 Videos
- CHEMISTRY OF NON-METALLIC ELEMENTS AND THEIR COMPOUNDSBook:MTG-WBJEEChapter:CHEMISTRY OF NON-METALLIC ELEMENTS AND THEIR COMPOUNDSExercise:WB JEE PREVIOUS YEARS QUESTIONS(CATEGORY 3 : One or More Option Correct Type)
Explore 1 Video
- ENVIRONMENTAL CHEMISTRY Book:MTG-WBJEEChapter:ENVIRONMENTAL CHEMISTRY Exercise:WB JEE Previous Years Questions ( CATEGORY 1 : Single Option Correct Type )
Explore 2 Videos
Similar Questions
Knowledge Check
Which of the following compounds is square planar and does not have any unpaired electron ?
ANi(CO)4B[Ni(H2O)6]2+C[NiCl4]2−D[Ni(CN)4]2−Which one of the following compounds is not planar
AC2H2>SO2>CH4>H2O>NH3BH2O>NH2>CH4>C2H2>SO2CH_2O gt NH_3 gt CH_4 gt SO_4gt C_2H_2DC2H2>SO2>CH4>NH3>H2OWhich of the following compound or ion is planar?
ASF−5BSF4CSOF4DSF2
Which one of the following compounds is not planar?
View SolutionWhich one of the following compound is not a planar ?
View SolutionWhich of the following is a square planar complex?
View SolutionWhich of the following is not square planar ? .
View SolutionWhich of the following is not square planar ?
View Solution

