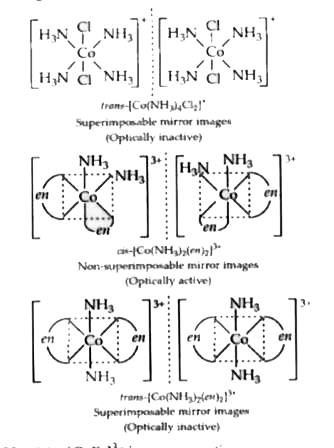
Which complex is likely to show optical activity?
A
Trns-[Co(NH3)4Cl2]+
B
[Cr(H2O)6]3+
C
Cis -[Co(NH3)3(en)2]3+
D
Trans[Co(NH3)2(en)2]3+
Text Solution
Octahedral complexes of the type Ma6 do not show geometrical and optical isomerism.
|
Topper's Solved these Questions
- COORDINATION COMPOUNDSBOOK - MTG-WBJEECHAPTER - COORDINATION COMPOUNDSEXERCISE - WBJEE/WORKOUT (Category 2: Single Option Correct Type)
13 Videos
- COORDINATION COMPOUNDSBOOK - MTG-WBJEECHAPTER - COORDINATION COMPOUNDSEXERCISE - WBJEE/WORKOUT (Category 3: One or More than one Option Correct Type)
7 Videos
- CHEMISTRY OF NON-METALLIC ELEMENTS AND THEIR COMPOUNDSBOOK - MTG-WBJEECHAPTER - CHEMISTRY OF NON-METALLIC ELEMENTS AND THEIR COMPOUNDSEXERCISE - WB JEE PREVIOUS YEARS QUESTIONS(CATEGORY 3 : One or More Option Correct Type)
1 Video
- ENVIRONMENTAL CHEMISTRY BOOK - MTG-WBJEECHAPTER - ENVIRONMENTAL CHEMISTRY EXERCISE - WB JEE Previous Years Questions ( CATEGORY 1 : Single Option Correct Type )
2 Videos
Similar Questions
Knowledge Check
One among the following complex ions will not show optical activity
A[Pt(Br)(Cl)(I)(NO2)(C5H5N)(NH3)]−Bcis−[Co(en)2Cl2]+C[Co(en)(NH3)2Cl2]+D[Cr(NH3)4Cl2]+Which of the following complexes does not show optical activity ?
A[Co(EDTA)]−B[Pt(bn)2]2+C[Pt(pn)2]2+D[Pt(en)2]2+Which of the following complex will show optical activity ?
Atrans−[Co(NH3)4Cl2]+B[Cr(H2O)6]3+Ccis−[Co(NH3)2(en)2]3+Dtrans−[Co(NH3)2(en)2]3+
Which of the following complex entity can show optical activity?
View SolutionWhich one of the following shows optical activity ?
View SolutionThe complex that shows optical activity is :
View SolutionThe complex that shows optical activity is :
View SolutionThe complex that can show optical activity is :
View Solution

